Diethyl Ether Phase Diagram
Consider first a two component water dimethyl ether system.
Diethyl ether phase diagram. Normally we represent these phases in a triangular diagram where the apices of the triangle represent the three pure components and the distance from an apex is a measure of it s concentration. If we consider. Text g ml so is the top organic layer in the funnel. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines.
116 c tci d3479 116 c alfa aesar 116 c ou chemical safety data no longer updated more details 116 3 c jean claude bradley open melting point dataset 21939 116 c jean claude bradley open melting point dataset 13163 15719 6838 116 c alfa aesar 16767 33224 38990 40976 178 176 f 116 6667 115 5556 c wikidata q202218 177 f 116 1111 c wikidata q202218. In this case the differential solubility in the immiscible solvents allows the two phase liquid system to be used to separate solutes using a separatory funnel method. If it s a glow motor it should run on a methanol oil mix but will take a diesel mix with ether. A ternary phase diagram shows the phases of a three component system.
The diagrams are drawn by plotly javascript open source graphing library. Figure 4 16 shows a diagram of an aqueous solution being extracted twice with diethyl ether. Ether c2h5 2o or c4h10o cid 3283 structure chemical names physical and chemical properties classification patents literature biological activities. Alcohols methanol ethanol can be used.
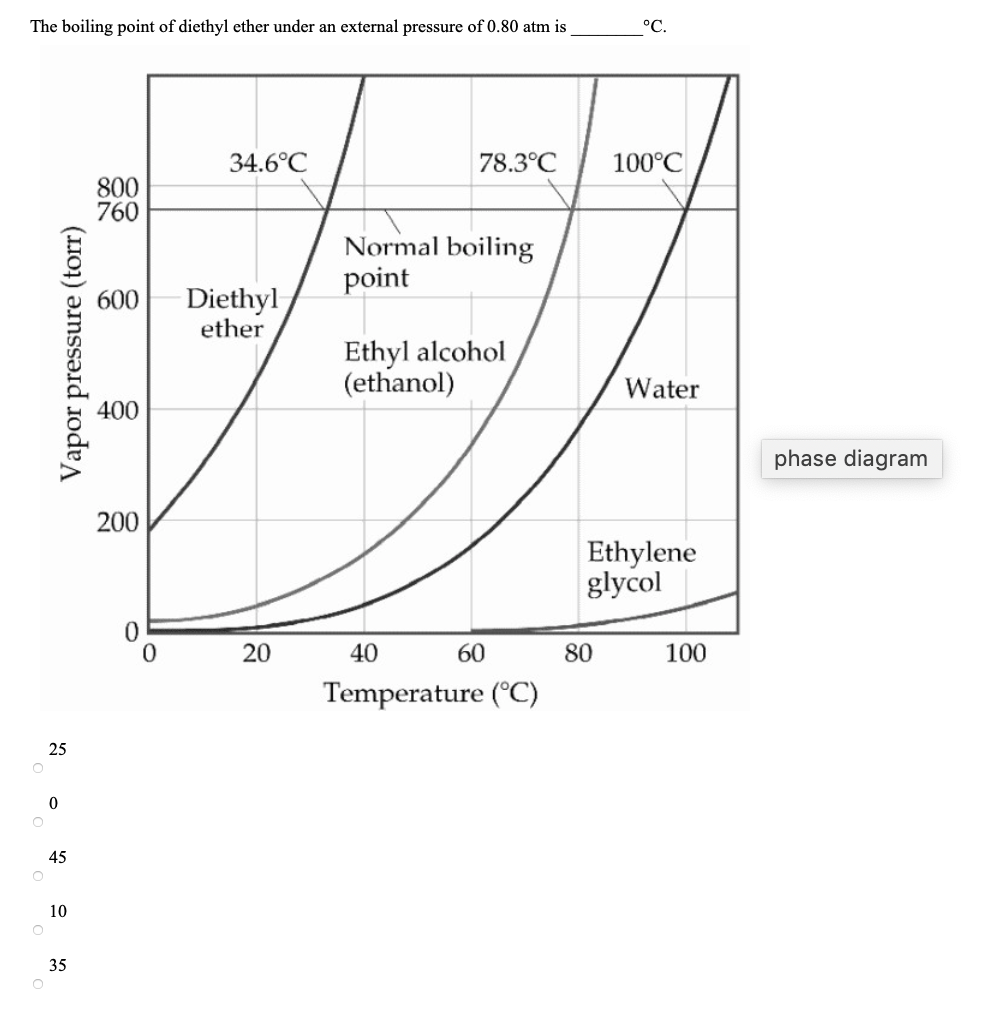
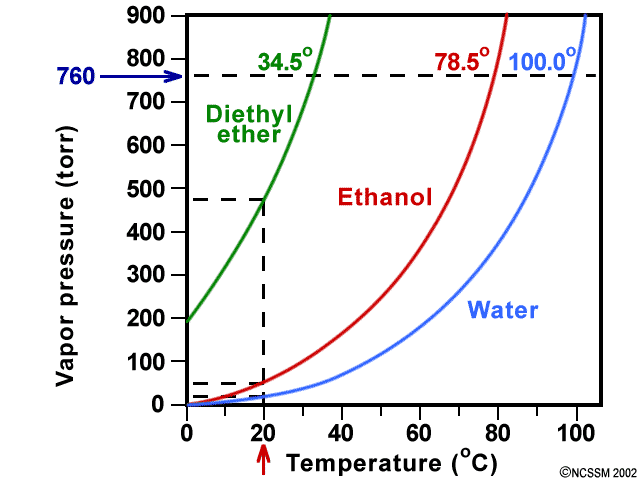
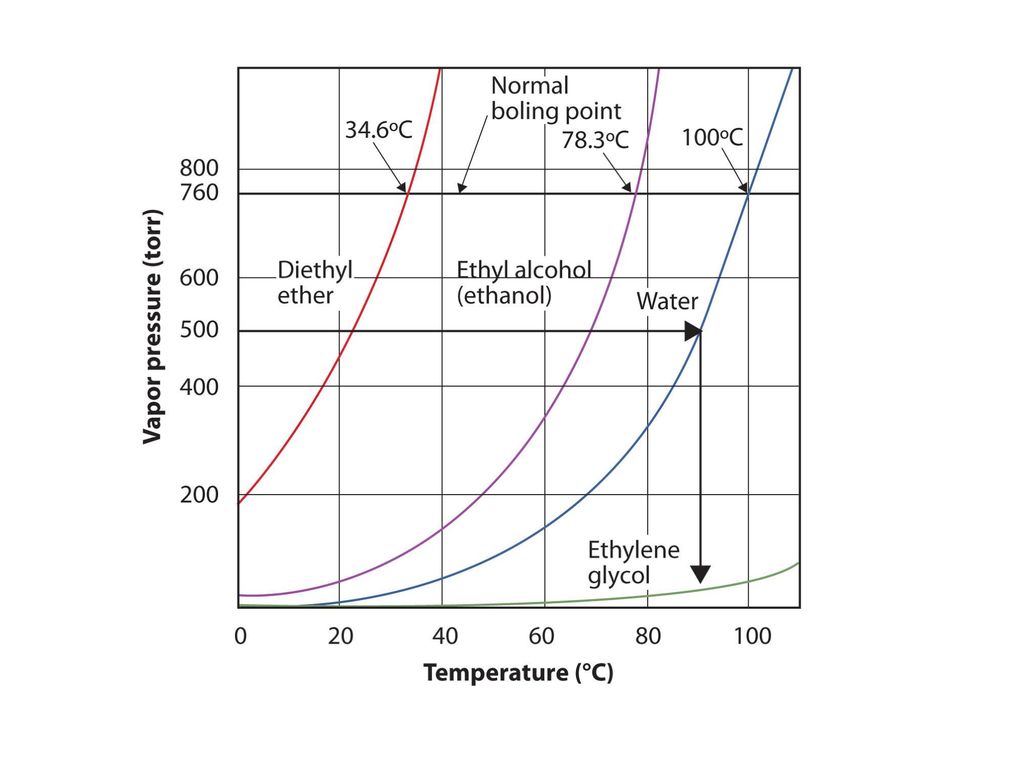
It was formerly used as a general anesthetic until. Another case that is commonly used in the organic chemistry laboratory is the combination of diethyl ether and water. Diethyl ether can be used but it is very flammable and volatile. How much energy is needed to vaporize 75 0 g of diethyl ether c4h10o at its boiling point 34 6 c given that δhvap of diethyl ether 26 5 kj mol.
Multiple extractions of an aqueous layer when the organic layer is on the top. Acetic acid a carboxylic acid can be used usually as a small percentage component of the system since it is corrosive non volatile very polar and has irritating vapors. If it s a diesel it needs the proper mix with ether. Select the true statement regarding the enthalpy of phase changes the enthalpy of vaporization is endothermic.