Diethyl Ether Hydrogen Peroxide
Diethyl ether is typically supplied with trace amounts of the antioxidant bht 2 6 di tert butyl 4 methylphenol which reduces the formation of peroxides.
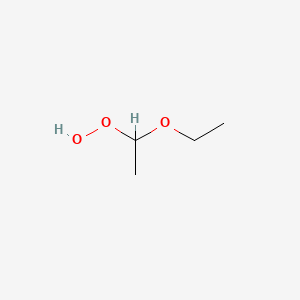
Diethyl ether hydrogen peroxide. Many organic chemicals form peroxides in presence of atmospheric oxygen and sometimes ultraviolet light. A typical example is the diethyl ether peroxide. Diethyl ether peroxide also known as ethylidene peroxide is a polymerization product of diethyl ether hydroperoxide. Diethyl ether hydroperoxide can be formed by.
If you prevent the formation of diethylether hydroperoxides by. Diethyl ether peroxide c4h10o3 cid 126387 structure chemical names physical and chemical properties classification patents literature biological. Storing diethylether in a brown bottle over sodium hydroxide. Diethyl ether hydroperoxide and its condensation products are blamed for the explosive organic peroxides that slowly form upon exposure of diethyl ether to ambient air and temperature conditions.
Diethyl ether is prone to peroxide formation and can form explosive diethyl ether peroxide. As they combine unstably bound oxygen together with hydrogen and carbon in the same molecule organic peroxides catch fire easily and burn rapidly and intensely.