Ethanol Diethyl Ether Boiling Point
Ether peroxides have a higher boiling point than ether and are contact explosives when dry.
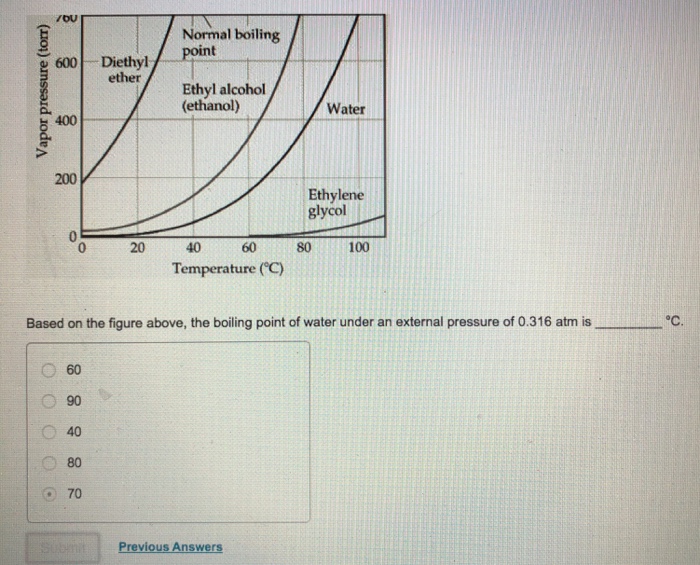
Ethanol diethyl ether boiling point. The diffusion of diethyl ether in air is 9 18 10 6 m 2 s 298 k 101 325 kpa. It is the most common member of a class of chemical compounds known generically as ethers. Diethyl ether also known as ether and ethoxyethane is a clear colorless and highly flammable liquid with a low boiling point and a characteristic smell. The melting point of dimethyl ether is 141 c.
Chemsrc provides diethyl ether cas 60 29 7 msds density melting point boiling point structure formula molecular weight etc. Ether is synthesized by the dehydration of ethanol using sulphuric acid. Diethyl ether has the formula ch 3 ch 2 o ch 2 ch 3. Ethanol has a characteristic alcoholic odor.
Boiling point of ethanol is 78 37 c. Boiling point of dimethyl ether is 24 c. Synthesis of diethyl ether c 2 h 5 2 o. Articles of diethyl ether are included as well.
Citation needed ether is sensitive to light and air tending to form explosive peroxides. Dimethyl ether is a colorless gas at room temperature. Ethanol is a colorless liquid at room temperature with high volatility. Ether c2h5 2o or c4h10o cid 3283 structure chemical names physical and chemical properties classification patents literature biological activities.
For example dimethyl ether and ethanol both having the molecular formula c 2 h 6 o are completely soluble in water whereas diethyl ether and 1 butanol both c 4 h 10 o are barely soluble in water 8 g 100 ml of water. It is an isomer of butanol.