Ethanol Vs Dimethyl Ether Boiling Point
For example dimethyl ether and ethanol both having the molecular formula c 2 h 6 o are completely soluble in water whereas diethyl ether and 1 butanol both c 4 h 10 o are barely soluble in water 8 g 100 ml of water.
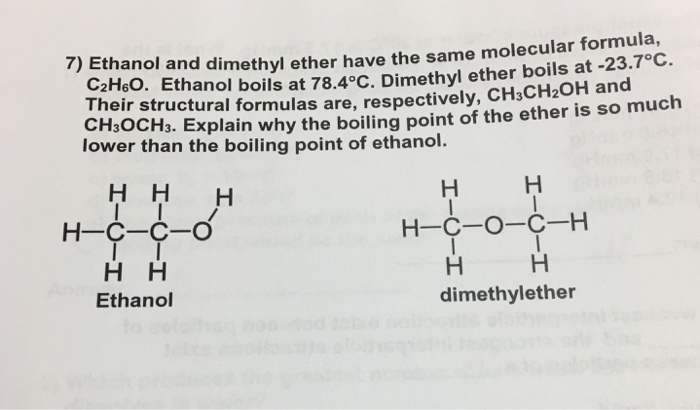
Ethanol vs dimethyl ether boiling point. Ethanol is an alcohol having the chemical formula c 2 h 5 oh. As a result alcohols have boiling points that are much higher than alkanes with similar molecular weights. Yet the boiling point of ethanol is 78 7 ᵒ c whereas the boiling point of dimethyl ether is 24 9 ᵒ c. The melting point of ethanol is 114 1 c.
Ethanol vs dimethyl ether. For example ethanol ch3ch2oh and dimethyl ether ch3och3 are structural isomers but have different boiling points. The melting point of dimethyl ether is 141 c. Ethanol has an oxygen bonded with a hydrogen meaning that one of the forces is hydrogen bonding whereas dimethyl ether s intermolecular forces are dipole dipole.
The simplest ether it is a colorless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applications it is an isomer of ethanol. Ethanol will have a greater surface tension than dimethyl ether surface tension will depend on the intermolecular forces in a compund. Dimethyl ether is an ether. Why solubility of alcohol in water decreases with increasing molecular mass.
Ethanol is an alcohol. Dimethyl ether dme also known as methoxymethane is the organic compound with the formula ch 3 och 3 simplified to c 2 h 6 o. The boiling point of ethanol for example is 78 5ºc whereas propane with about the same molecular weight boils at 42 1ºc. So since hydrogen bonds dipole diple ethanol will have greater suface tension.
Alcohol is soluble in water due to formation of inter molecular hydrogen bond with water molecule. Question from student questions chemistry.