Ethanol Dehydration To Dimethyl Ether
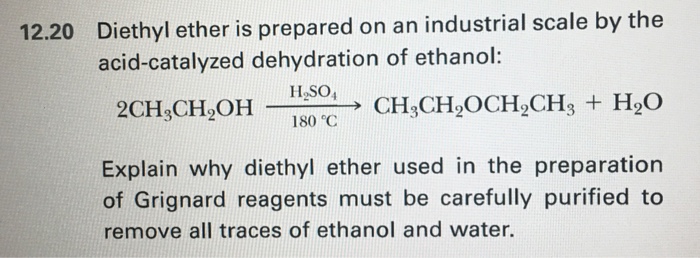
Dehydration of ethanol to diethyl ether dee was studied over cu fe zsm 5 catalysts over the temperature range of 160 220 c.
Ethanol dehydration to dimethyl ether. Dimethyl ether dme has been considered a potential and promising energy alternative for petroleum subproducts. Dimethyl ether dme as a solution to environmental pollution and diminishing energy supplies can be synthesized more efficiently compared to conventional methods using a catalytic distillation column for methanol dehydration to dme over an active and selective catalyst. Chemical engineering and processing. Peng lu guohui yang yuki tanaka noritatsu tsubaki ethanol direct synthesis from dimethyl ether and syngas on the combination of noble metal impregnated zeolite with cu zno catalyst catalysis today 10 1016 j cattod 2013 10 042 232 22 26 2014.
The key difference between ethanol and dimethyl ether is that the ethanol is a colorless liquid at room temperature which has high volatility whereas dimethyl ether is a colorless gas at room temperature further ethanol common name is ethyl alcohol is an alcohol while dimethyl ether is an ether. If ethanol is dehydrated to ethene in presence of sulfuric acid at 433 k but as 410 k ethoxyethane is the main product. First published on 24th october 2017. Furthermore dme can be considered a cleaner fuel than diesel.
Due to its good burning characteristics and to its high cetana content which is superior to that of diesel. Metal loaded samples were prepared by wet impregnation and characterized using specific surface area measurements x ray diffraction and temperature programmed desorption of nh 3 the presence of fe on zsm 5 did not change the acidity of zsm 5 but did decrease its. The first and most straightforward is to mix ethanol with a strong acid typically sulfuric acid h2so4. Methyl acetate ma was formed by dme carbonylation over the h mordenite catalyst.
Process intensification 2013 73 144 150. The acid dissociates in the aqueous like environment produci. From laboratory experiments to simulation studies of methanol dehydration to produce dimethyl ether part i. Dme can be produced by dehydration reaction of methanol.
Here we show a strategy of ethanol synthesis from co 2 dimethyl ether dme. There are two ways to make diethyl ether ch3ch2och2ch3 from ethanol ch3ch2oh. The synthesis of ethanol from cheap and renewable co 2 is of great importance but the state of the art routes encounter difficulties especially in reaction selectivity and activity. Ethanol was directly synthesized from dimethyl ether dme and syngas with the combined h mordenite and cu zno catalysts that were separately loaded in a dual catalyst bed reactor.