Ethyl Ether Dipole Moment
Dipole moments last updated.
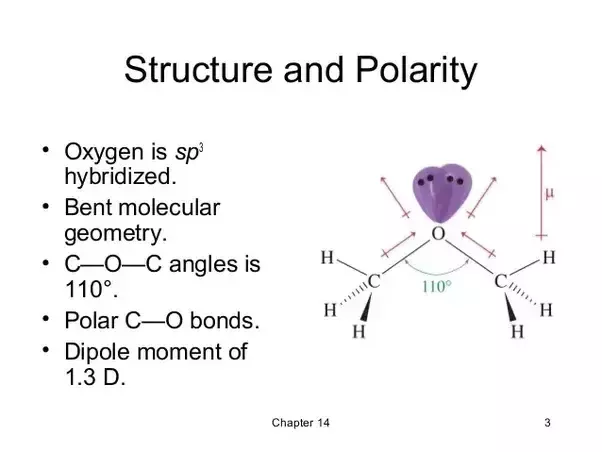
Ethyl ether dipole moment. As a result the c o bond dipoles partially reinforce one. 0 24 cp at 20 c. It should therefore have a very small but nonzero dipole moment and a very low boiling point. It contains two polar c o single bonds oriented at about a 109 angle to each other in addition to relatively nonpolar c h bonds.
Why are the dipole dipole forces in ethanol stronger than those in ethyl ether. Like ethyl ether ethanol is a polar molecule and will experience dipole dipole interactions. Dipole moments of individual conformers rotational isomers are given when they have been measured. 1 15 d at 20 c.
Solubility of water in ethyl ether. As a result the c o bond dipoles partially reinforce one. Dipole moment this page provides a list of dipole moment for about 800 molecules including organic and inorganic. The especially strong intermolecular forces in ethanol are a result of a special class of dipole dipole forces called hydrogen bonds.
It was formerly used as a general anesthetic until. The following table 1 lists the dipole moments of more common chemical substances. It contains two polar c o single bonds oriented at about a 109 angle to each other in addition to relatively nonpolar c h bonds. Save as pdf page id 10976.
The conformers are designated as gauche trans axial etc. Nuclear quadrupole coupling. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. Ethyl methyl ether has a structure similar to h 2 o.
It should therefore have a very small but nonzero dipole moment and a very low boiling point. The experimental data shown in these pages are freely available and have been published already in the ddb explorer edition the data represent a small sub list of all available data in the dortmund data bank for more data or any further information please search the ddb or contact ddbst. Dipole moment of diethyl ether. In some cases an average value obtained from measurements on the bulk gas is.
Ethyl methyl ether has a structure similar to h 2 o. Along with the dipole moment the length of the dipole is shown. 17 06 dyn cm at 20 c.