Diethyl Ether Water Polar
Diethyl ether is polar relative to other molecules such as hexane.
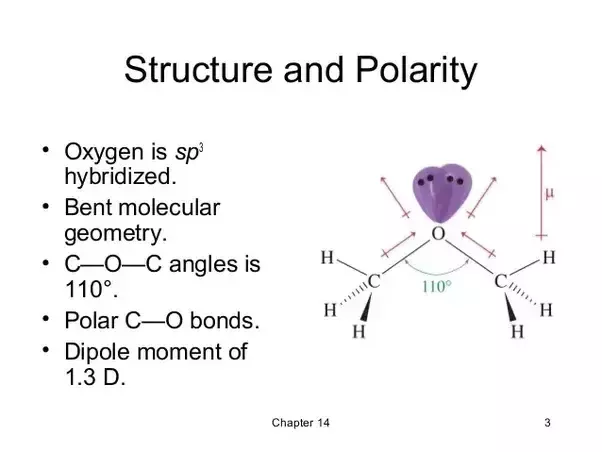
Diethyl ether water polar. It has a dipole moment for reasons already given it is a molecule with bent geometry. It also has nonpolar side chains which are not attracted to the polar molecule water. Could you please help for the tips i would like to know whether ethyl ether or other name diethyl ether is the non polar is because i am interested to use it for extraction of wet algae. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines.
Cid 3283 ether. But in practice it is considerably less polar than water and in fact is mostly insoluble in it. Topological polar surface area. Diethyl ether cannot participate in hydrogen bonding with water.