Why Is Diethyl Ether Used For Extraction
When utilizing extraction solvents for liquid liquid extraction two solvents must be used.
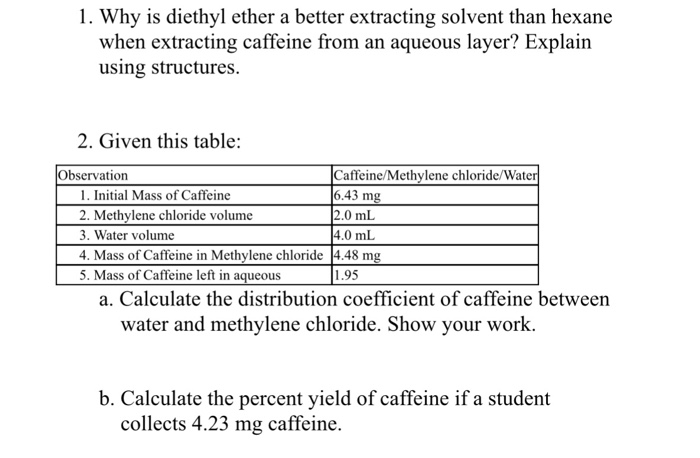
Why is diethyl ether used for extraction. For extraction of organic solvents generally diethylether is used because organic substances are generally soluble in ether and ether has got a low boiling point making its removal after the. Text ml portion of diethyl ether to the separatory funnel. There is no need to wash the funnel in between extractions. Text g ml so is the top organic layer in the funnel.
Diethyl ether is considered a good organic extracting solvent because it has a low polarity according to the university of alberta s organic web chem. Polarity is a relative term ether is considered nonpolar and water polar. Why does the product precipitate out of the isopropanol but the starting material is soluble in the solvent. Add a fresh 25.
Even though it is a very stable ion it rearranges to another carbocation. Anhydrous diethyl ether ch3ch2och2ch3 and petroleum ether which is a mixture of pentane ch3ch2ch2ch2ch3 and hexane ch3ch2ch2ch2ch2ch3. Why are three layers observed sometimes. The extraction is repeated two to three times or perhaps more times if the compound has a low partition coefficient in the organic solvent.
One is usually water or water based and the other an organic solvent. The main route of exposure is inhalation. Diethyl ether has a density less than 1. In part a the reaction fails if diethyl ether is used instead of isopropyl alcohol.
Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. In part b a tertiary benzylic carbocation is initially formed. The most common pair of extraction solvents used is diethyl ether often referred to as simply ether and water. It was formerly used as a general anesthetic until.
Because of its characteristics diethyl ether was widely used in many countries as an anesthetic agent but was then replaced by other substances in the 1960s. Stopper the funnel invert and shake with venting then allow the layers to separate. Thus diethyl ether and ethyl acetate which are both less dense than the dilute solutions that are usually used for extraction form the top layer while dichloromethane and chloroform form the bottom layer currently both of them are not used in chem 30bl or chem30cl due to safety concerns. Return the aqueous layer to the separatory funnel.
The fact that two phases are observed upon adding one to the other is a consequence of their different polarities. When talking about ether two kinds are used in lipid extraction.