Mg Diethyl Ether Reaction
Repeated punctures will likely result in decreased performance of product.
Mg diethyl ether reaction. 10 20 time min fig. A grignard reaction involves the reaction of an alkyl or aryl halide with magnesium metal to form an alkylmagnesium halide. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. To the residue in the reaction vessel diethyl ether 6 ml and 2 2 biquinoline 0 5 mg in 100 al diethyl ether were added.
The adding of diethyl ether in the mixture is works for the similar function which make sure the solvent is free from water. In a separate oven dried vial add bromobenzene 330 mg 2 1 mmol and 0 7 ml of anhydrous diethyl ether and transfer 0 1 ml of this solution into the. This weekend there is the model engineering show at alexandra palace. Packaging 4 25 100 800 ml in sure seal 8 l in kilo lab the 25 ml sure seal bottle is recommended as a single use bottle.
The flask is fitted with a reflux condenser and the mixture is warmed over a water bath for 20 30 minutes. The rate of reaction of organic halides with magnesium is proportional to organic halide concentration and magne sium surface area. It was formerly used as a general anesthetic until. Ethylmagnesium bromide solution 3 0 m in diethyl ether can be used for copper i catalyzed allylic substitution reaction.
Weigh magnesium powder 50 mg 2 mmol and add it to your reaction vessel. 33 the tendency of alkyl halide to form quaternary ammonium salts can be largely avoided but the solubilities of mg r x compounds are only 0 1 0 9 m compared to 2 4 m in diethyl ether. I m sure one or more of the exhibitors or clubs there will either sell it or know who does. Reactions in triethylamine give high yields for primary alkyl chlorides or bromides 80 90 but lower for branched halides or iodides.
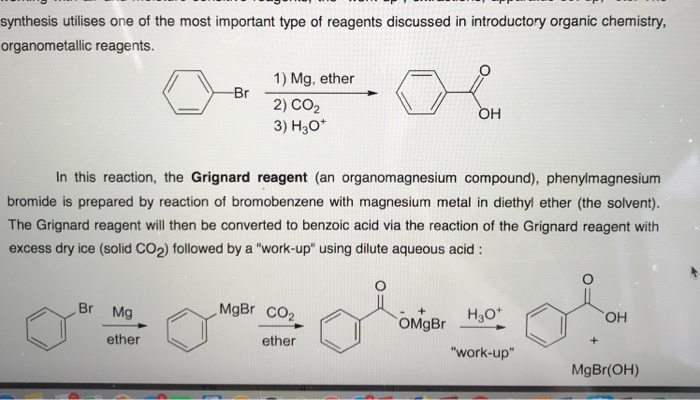
Grignard reagents are made by adding the halogenoalkane to small bits of magnesium in a flask containing ethoxyethane commonly called diethyl ether or just ether. Besides diethyl ether is easily removed from the reaction mixture since it has a low boiling point of 36 c. Ether is used as a solvent because it is aprotic and can solvate the magnesium ion. A mixture of bromobenzene and diethyl ether was prepared in the dropping funnel.
Chlorocyclohexane mg diethyl ether kind reaction.