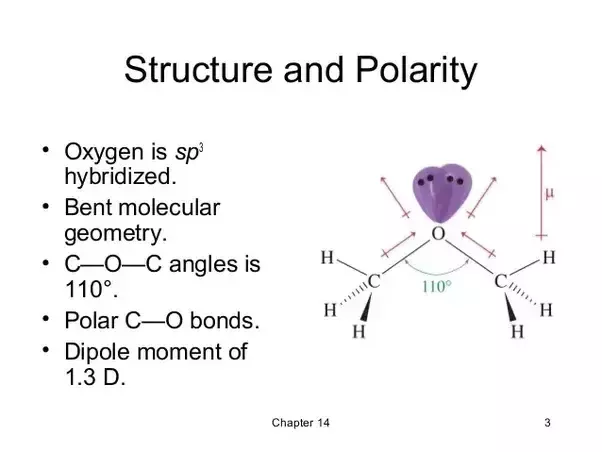
Dimethyl Ether Molecular Geometry
And thus to a first approx h c h c o c o c h 109 5.
Dimethyl ether molecular geometry. The ce ch3 functional group is many times heavier than the h atom both act as side atoms. Because oxygen bears 2 lone pairs which as non bonding entities tend to lie closer to the oxygen atom the low pairs may tend to compress c o c. The electronic geometry on each non hydrogen atom is tetrahedral to a first approx. Idealized geometry with and without h atom s frag 17 c1 6 2 386697 0 000000 0 394384 c2 6 1 187650 0 000000 0 540118 o3 8 0 000000 0 000000 0 239004 c4 6 1 187650 0 000000 0 540118.
Dimethyl ether dme also known as methoxymethane is the organic compound with the formula ch 3 och 3 simplified to c 2 h 6 o. The bond angle in dimethyl ether is greater due to the following reason. Solved che 1502 10 to 13. Consider the reaction below.
Any leak can be either liquid or vapor. Arrange the following compounds in increasing order of their. It is shipped as a liquefied gas under its vapor pressure. Contact with the liquid can cause frostbite.
Dimethyl ether ch3och3 and ethanol ch3ch2oh are. Ether c2h5 2o or c4h10o cid 3283 structure chemical names physical and chemical properties classification patents literature biological activities. Gaussian r 03 level of theory. The actual values are the preserve of experiment.
C 2v optimization program. Shields demonstrates how to figure out whether or not dimethyl ether has an overall molecular dipole moment i e is polar or nonpolar. We deal with dimethyl ether h 3c o ch 3. The simplest ether it is a colorless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applications it is an isomer of ethanol.
Dimethyl ether is a colorless gas with a faint ethereal odor. Electron geometry and molecular geometry of ch3och3. 138 c ou chemical safety data no longer updated more details 138 c jean claude bradley open melting point dataset 15735 141 5 c jean claude bradley open melting point dataset 20330 141 c synquest 141 5 c foodb fdb006938 141 c synquest 2107 1 01 141 c sigma aldrich aldrich 295299. Indicate whether the compounds in each set are.
Apr 24 2020 contributor. So the effect of lone pair lone pair repulsion in ce h2o is pronounced in ce h2o whereas due to heavy molecular weight the repulsive forces aren t able to fully force. It is easily ignited.