Dimethyl Ether Boiling Point Vs Ethanol

Dimethyl ether dme also known as methoxymethane is the organic compound with the formula ch 3 och 3 simplified to c 2 h 6 o.
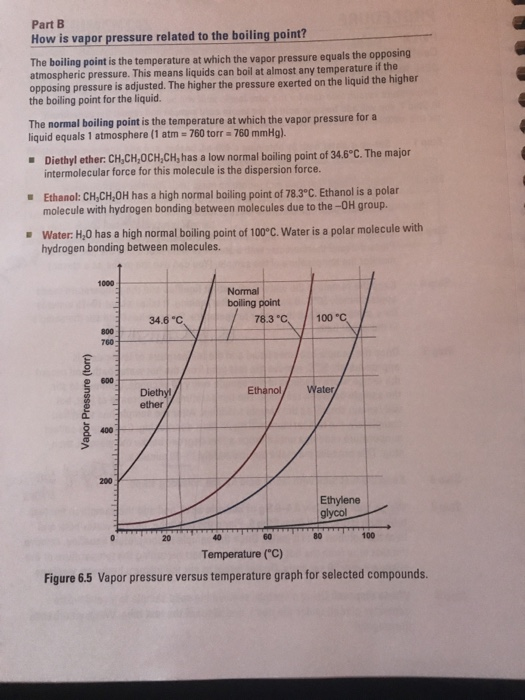
Dimethyl ether boiling point vs ethanol. Dimethyl ether is an ether. The boiling point of ethanol is much higher than the boiling point of. Ethanol vs dimethyl ether. Ethanol is an alcohol.
The boiling point of any liquid can be defined as the minimum temperature at. Ethanol is an alcohol having the chemical formula c 2 h 5 oh. 1 solubility in water 2 rate of evaporation homework equations the attempt at a solution 1 due to the oxygen being bonded to 2 carbon atoms none of the hydrogen atoms in the dimethyl ether molcule have a dipole. What are the boiling points of ethanol dimethyl ether propane water and methyl alcohol.
This means that diethyl ether cannot form. The melting point of dimethyl ether is 141 c. B of stronger dipole dipole interactions. Ethanol and dimethyl ether have the same molecular formula c2h6o but the boiling point of ethanol.
Dimethyl ether is an ether compound having the chemical formula c 2 h 6 o. The melting point of ethanol is 114 1 c. Dimethyl ether and ethanol has same molecular weight but boiling point of ethanol is greater than dimethyl ether cause of this is that dimethyl ether. For example ethanol ch3ch2oh and dimethyl ether ch3och3 are structural isomers but have different boiling points.
Explain the difference in the following properties of dimethyl ether and ethanol.