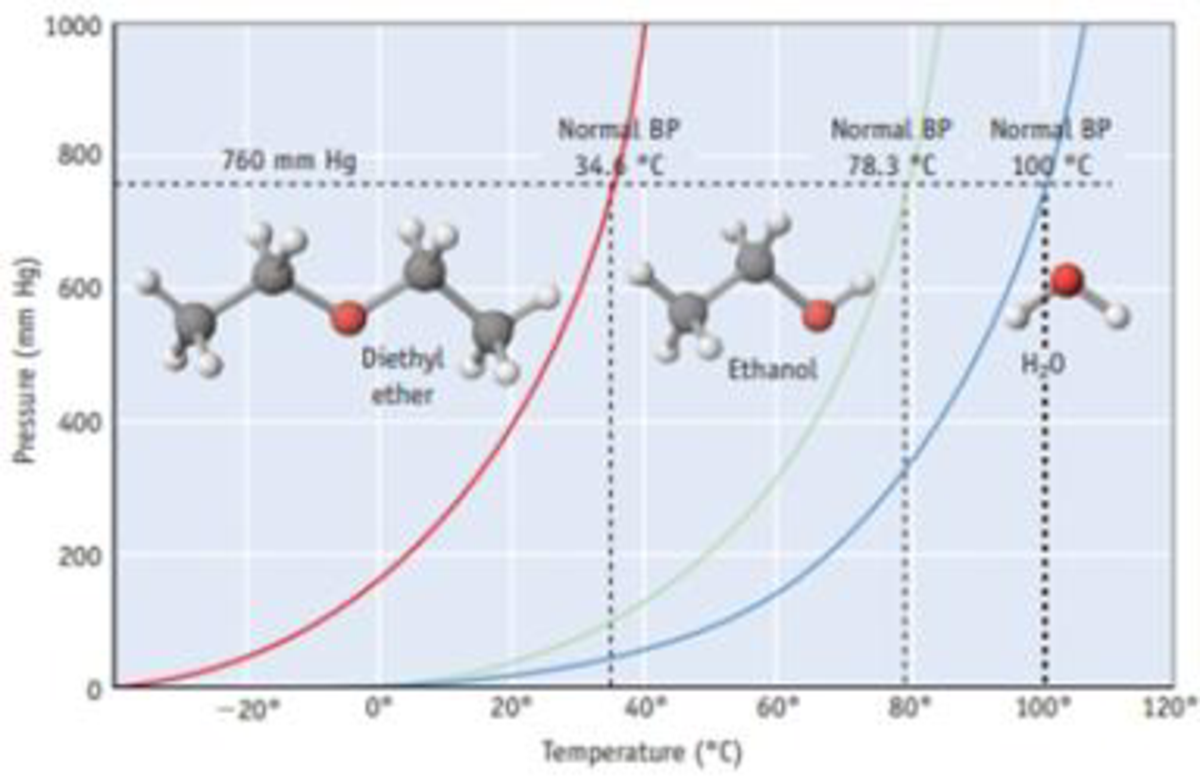
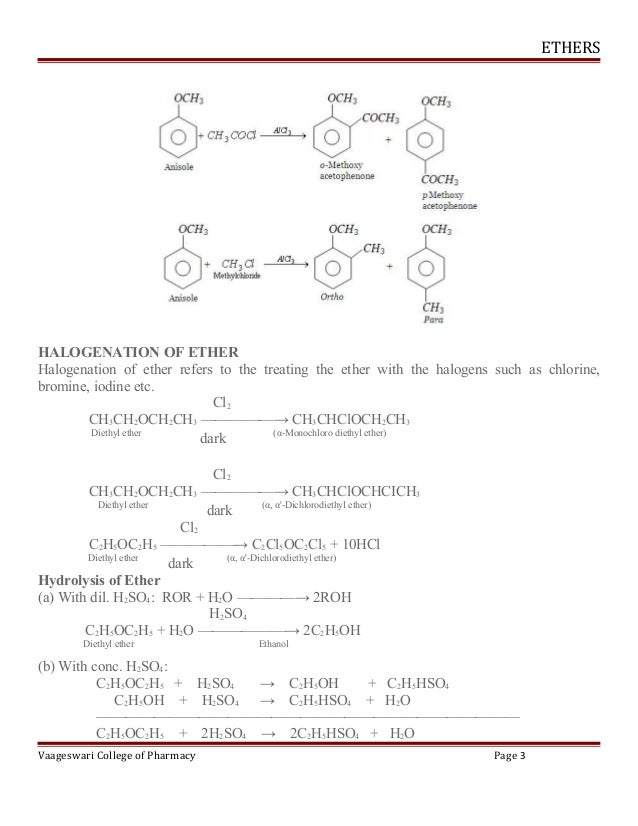
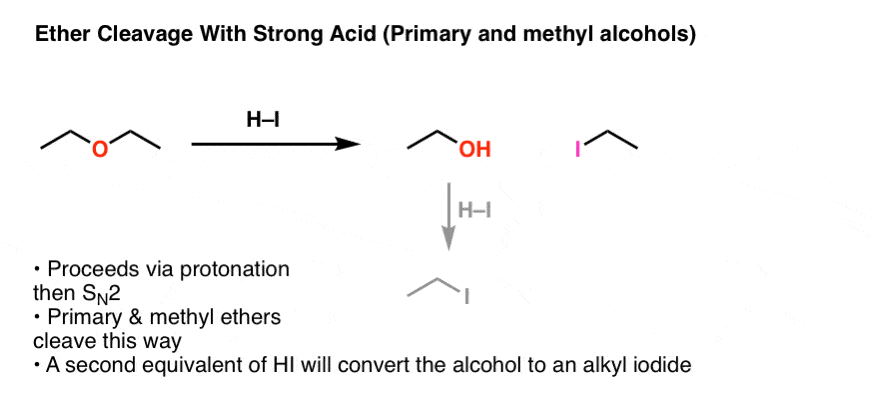
Formation Of Diethyl Ether From Ethanol Is Based On
In the present work mechanistic pathways and kinetics of catalytic dehydration of ethanol were investigated in a closed batch reactor for the formation of diethyl ether and ethylene over the synthesized nio loaded hzsm 5 in the range of 160 240 c.
Formation of diethyl ether from ethanol is based on. It is important to note that the attack of another ethanol molecule and the liberation of water happen synchronously. The synthesis of ethanol from cheap and renewable co 2 is of great importance but the state of the art routes encounter difficulties especially in reaction selectivity and activity. Ethanol is currently produced via the catalytic hydration of ethylene or fermentation of foods. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis.
No significant negative impact of water over diethyl ether. Ce ch3 ch2 oh2 ch3 ch2 oh ch3 ch2 oh ch2 ch3 h2o finally the oxygen which is now part of a protonated ether loses the proton to give the final product diethyl ether. Formation of diethyl ether from ethanol is based on a. Ncert dc pandey sunil batra hc verma pradeep errorless.
Ncert p bahadur iit jee previous year narendra awasthi ms chauhan. Vapor phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95. The acid dissociates producing hydrogen ions h. Formation of diethyl ether from ethanol is based on a.
Simulation of concentration profiles inside the microreactor channels. Download high res image 301kb download. Formation of diethyl ether from ethanol is based on a. There is no first or second.
The effect of the presence of water on reaction performance was also evaluated. Here we show a strategy of ethanol synthesis from co 2 dimethyl ether dme and h 2.