Dimethyl Ether Boiling Point Celsius

Dimethyl ether dme also known as methoxymethane is the organic compound with the formula ch 3 och 3 simplified to c 2 h 6 o.
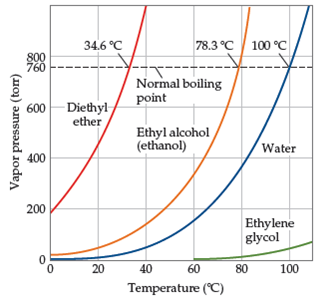
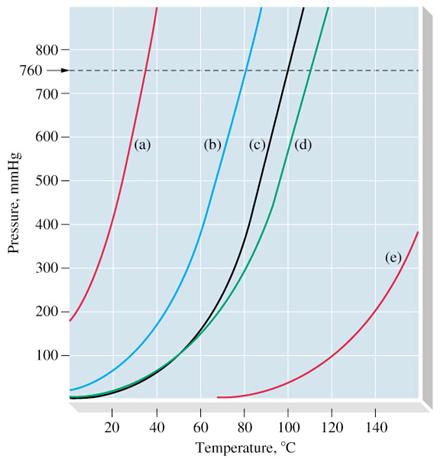
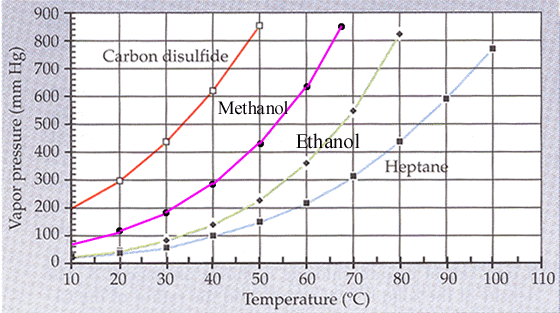
Dimethyl ether boiling point celsius. Match these boiling points with the correct compound. Flammable gas auto ignition temperature. It is easily ignited. 0 61 solubility in water g 100ml.
It is shipped as a liquefied gas under its vapor pressure. 141 5 c relative density water 1. 350 c explosive limits vol in air. The heat capacity and entropy heats of fusion and vaporization and the vapor pressure of dimethyl ether.
The density of gaseous dimethyl ether j. 3 4 26 7 octanol water partition coefficient as log pow. Justify why each boiling point is matched with that certain compound. Ether c2h5 2o or c4h10o cid 3283 structure chemical names physical and chemical properties classification patents literature biological activities.
1 4 dimethoxybenzene c8h10o2 cid 9016 structure chemical names physical and chemical properties classification patents literature biological activities. The boiling point of butane is 0 5 degrees celsius 133f is the boiling point of acetone the boiling point of methyl ethyl ether is 52 0 f and im sorry about the reasons i will search for it and may i can find it but just nitice that the first one is in celsius and the others are not so try to convert it and calculate which higher and which lower hope it helps. C 23 deg c and 78 5 deg c. Its vapors are heavier than air.
It can asphyxiate by the displacement of air. Stephenson and malanowski 1987. Contact with the liquid can cause frostbite. Diphenyl ether c6h5 2o or c6h5oc6h5 or c12h10o cid 7583 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information supplier lists and more.
Any leak can be either liquid or vapor. The simplest ether it is a colorless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applications it is an isomer of ethanol. Ch3ch2ch3 the formulas are written in such a way as to give you an idea of the structure the boiling points of these compounds are.