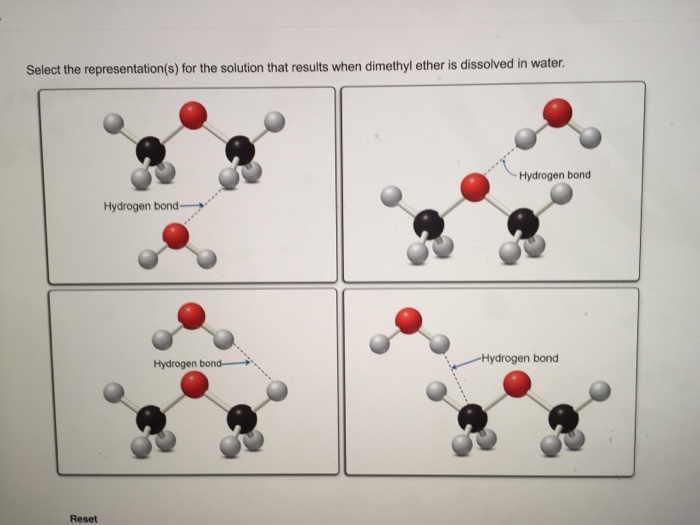
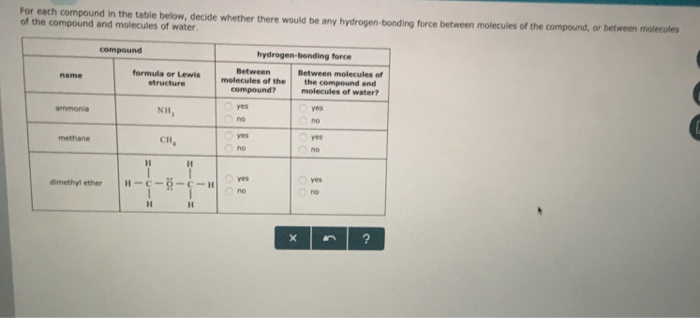
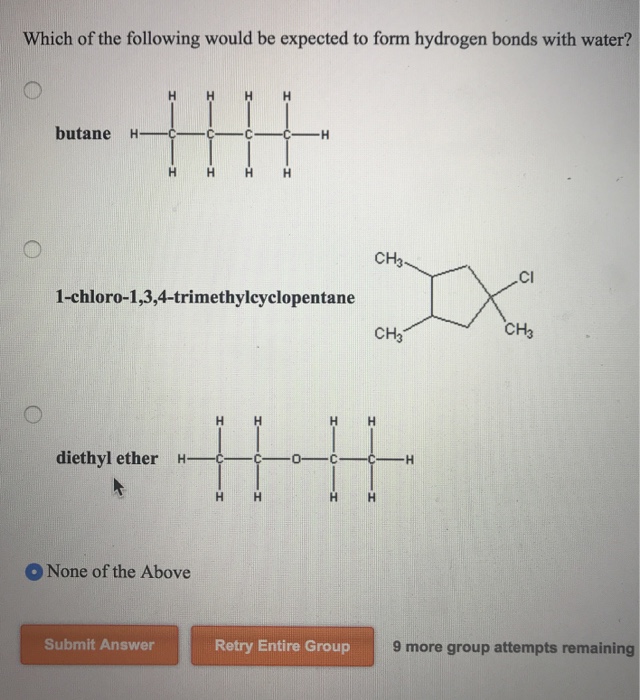
Dimethyl Ether And Water Hydrogen Bonding
Which of the following would be expected to form hydrogen bonds with water.
Dimethyl ether and water hydrogen bonding. Ethers containing up to 3. In which of the following pure substances would hydrogen bonding be expected. Nma and hydrogen of water. Each water molecule accepts two hydrogen bonds from two other water molecules and donates two hydrogen atoms to form hydrogen bonds with two more water molecules producing an open cagelike structure.
Dimethyl ether can not form h bonding only molecules where h is bonded directly to o n or f can you have h bonding as in intermolecular force in the case of dimethyl ether the o is bonded to each c and all of the h s are bonded to c. There will be more repulsion between bond pairs of ch 3 groups attached in ether than between bond pairs of hydrogen atoms attached to oxygen in water the carbon of ch 3 in ether is attached to three hydrogen atoms through σ bonds and electron pairs of these bonds add to the electronic charge density on carbon atom. The isotope effect and therefore the strength of the hydrogen bond network is more pronounced in water than in methanol. Hydrogen bonding only occurs between water molecules h2o or hf or hn.
Ch 3 och 3 dimethyl ether 46. An isotope effect is observed in water and methanol but not for dimethyl ether which cannot donate a hydrogen bond at its oxygen site. Ch 3 cn acetonitrile 41. You can contact our data protection supervisor by e mail.
The probabil ity of this interaction is higher with increasing dmso 0 0 6 1 2 1 8 2 4 g r o nma. Dimethyl sulfoxide dmso as a very important non proton polar solvent is widely used as solvent and reaction reagent 1 2 the study about binary mixtures of dmso and other co solvents especially water are of great significance in the fields of chemistry and biology hydrogen bonding is a special intermolecular force between dmso and water molecules which has an. он ethyl methyl ketone нн нн ethylamine h нн methyl propanoate. Dimethyl ether h f ch3 2 chloro 2 methylpropane ch3cch3 ci cyclohexane нн none of the above in which of the followi ing pure substances would hydrogen bonding be expected.
Vapor liquid equilibria of systems containing dichloromethane and gaseous components. Acetic acid acetone dimethyl ether n methylacetamide. The first peak at 2 74 å in figure 1a representing the correlation between oxy gen sites of nma and water arises due to the hydrogen bonding where nma molecule acts as an acceptor for the hydrogen of a water molecule. What is the intermolecular.