Separation Of Diethyl Ether And Water
Typical extraction solvents include ethyl acetate hexane chloroform methylene chloride and diethyl ether.
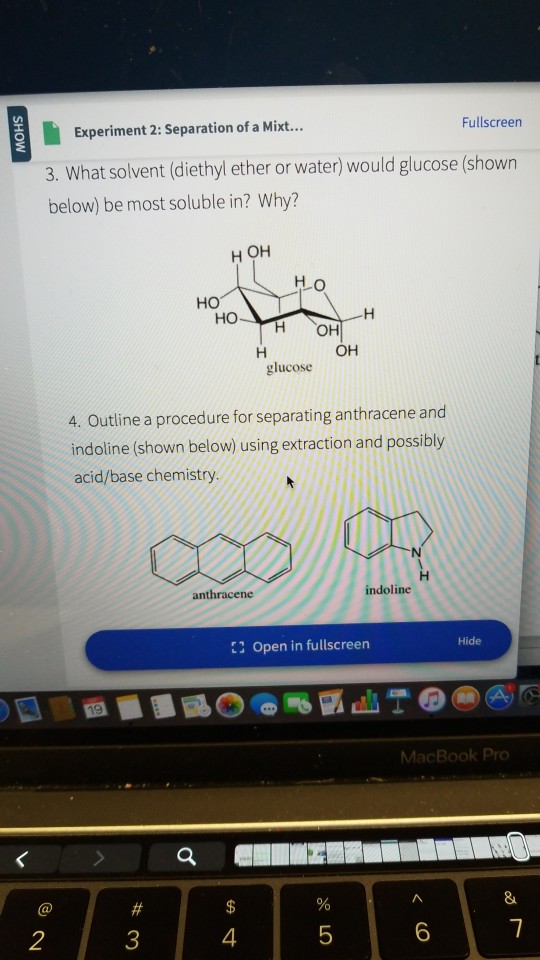
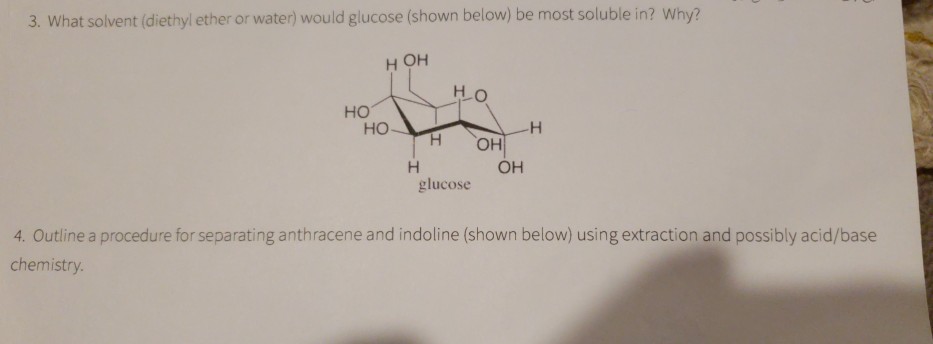
Separation of diethyl ether and water. Polarity is a relative term ether is considered nonpolar and water polar. Diethyl ether would be on top because it is non polar so it doesn t mix well with water and because it s less dense so it will float on top of water source s. This is because they are composed of many non polar c c and c h bonds and have. In their neutral covalent forms all of the compounds are soluble in a slightly polar organic solvent such as diethyl ether ch 3ch 2och 2ch 3 but are fairly insoluble in water.
All of these form crisp delineations between phases. Chem student 2 1. Both diethyl ether and benzene at first glance appear to be poor choices for extraction because caffeine is more soluble in water than in either solvent if a gram of caffeine dissolves in 46. Collect organic layer and washed several times with brine.
Diethyl ether and water think water whenever using aqueous solutions do not dissolve well in each other immiscible and will form layers in a container with the less dense liquid floating on top of the denser one. Separation and purification methods they have high solubilities in both aqueous and organic phases and can set up single phase systems i e nothing to separate or emulsions. The most common pair of extraction solvents used is diethyl ether often referred to as simply ether and water. Text ml water but 100.
To separate two compounds by taking advantage of differences in their acidity. Us2050600a us673630a us67363033a us2050600a us 2050600 a us2050600 a us 2050600a us 673630 a us673630 a us 673630a us 67363033 a us67363033 a us 67363033a us 2050600 a us2050600 a us 2050600a authority us united states prior art keywords ether solution diethyl ether treating line prior art date 1933 05 31 legal status the legal status is an assumption and is not a legal conclusion. Text ml of benzene caffeine is more soluble in water. The piece of equipment that is used for separating these layers or more.
The fact that two phases are observed upon adding one to the other is a consequence of their different polarities.