Higher Boiling Point Than Diethyl Ether
The diffusion of diethyl ether in air is 9 18 10 6 m 2 s 298 k 101 325 kpa.
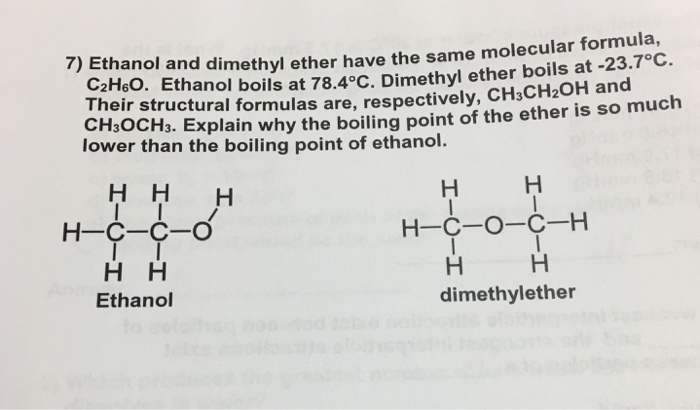
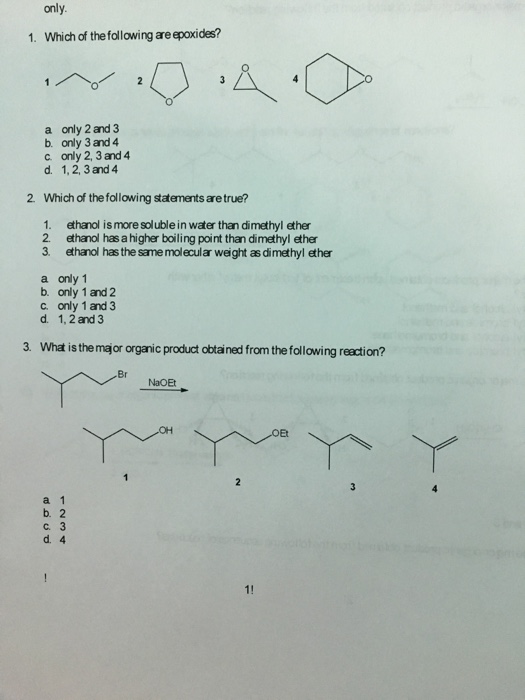
Higher boiling point than diethyl ether. For example ethanol ch3ch2oh and dimethyl ether ch3och3 are structural isomers but have different boiling points. Diethyl ether has no hydrogen bond which make it easier for molecules to break out when heated so it would have a low boiling point. Boiling is just about the bond between molecules of the substances or so i think. Diethyl ether and 1 butanol are similar in size number of electrons therefore their boiling points will be determined by polarity.
The boiling point of ethanol is much higher than the boiling point of. Both of them have simple molecular structure. Ether peroxides have a higher boiling point than ether and are contact explosives when dry. I don t think van der waals force london dispersion force have anything to do.
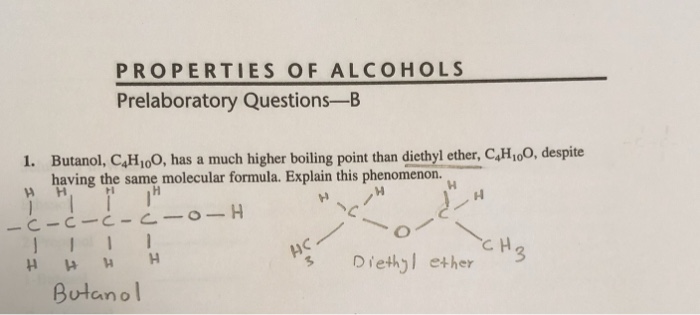
1 butanol also has. 1 butanol ch ch ch ch oh has a higher boiling point than diethyl ether ch ch och ch.