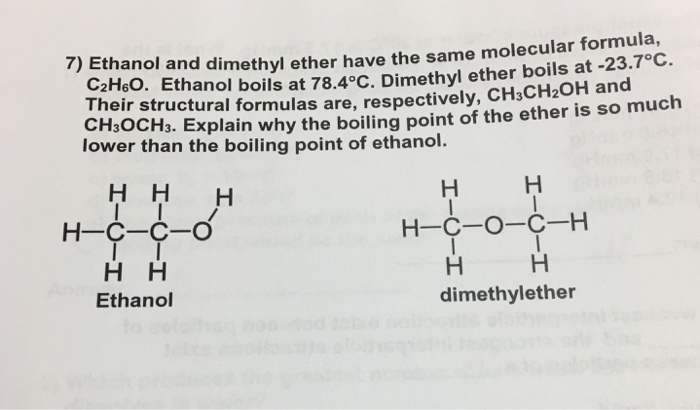
Ethanol Has Higher Boiling Point Than Diethyl Ether
Although dipole dipole forces and london dispersion forces also exist between ethyl alcohol molecules the strong hydrogen bonding interactions are responsible for the much higher normal boiling point compared to methyl ether.
Ethanol has higher boiling point than diethyl ether. On the other hand. For example ethanol ch3ch2oh and dimethyl ether ch3och3 are structural isomers but have different boiling points. Alcohols can form similar hydrogen bonds as shown in the figure below. Thus 1 butanol has the higher boiling point but they both are similarly soluble in water.
It has hydrogen bond which will hold the molecules together thus make it harder to separate them boiling is heating molecules so that the move out from the liquid. As a result alcohols have boiling points that are much higher than alkanes with similar molecular weights. The normal boiling point of ethyl alcohol is 78 5oc i e a liquid at room temperature. Ethanol has a characteristic alcoholic odor.
Actually morrison and boyd oversimplify the solubility difference and there is one. Diethyl ether has no hydrogen bond which make it easier for molecules to break out when. The melting point of dimethyl ether is 141 c. Butyl alcohol has the higher boiling point of course.
Water has an unusually high boiling point because of the hydrogen bonds between the h 2 o molecules. Boiling point of dimethyl ether is 24 c. Boiling points of alcohols. Dimethyl ether is a colorless gas at room temperature.
Boiling point of ethanol is 78 37 c. The boiling point of ethanol is much higher than the boiling point of. Ethanol is a colorless liquid at room temperature with high volatility.