Dimethyl Ether Polar Or Nonpolar
If you want to quickly find the word you want to search use ctrl f then type the word you want to search.
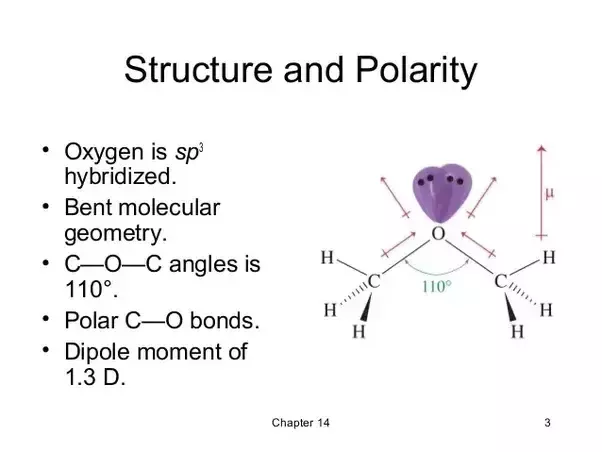
Dimethyl ether polar or nonpolar. Answer ch3och3 dimethyl ether is polar what is polar and non polar. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Each of the c o bonds are polarised as shown in the figure. Question is ch3och3 polar or nonpolar.
In addition to this the lone pair polarisation also contributes to its dipole moment. What is the formula of s ch3 2. Polar in chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. S ch3 2 is the formula for dimethyl sulfide analogous to dimethyl ether.
A nonpolar solvent will dissolve in a nonpolar solvent but not a polar solvent. Here s the best image i could fi. Dimethyl ether contains two methyl groups and has a bent structure.