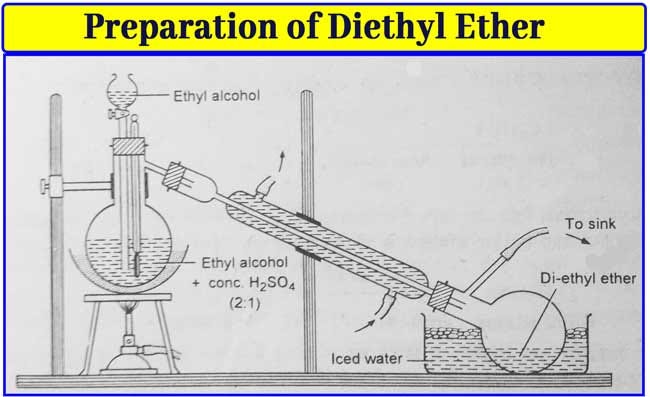
Diethyl Ether Peroxide Removal
Although it may be possible to stabilize isopropyl ether in other ways the absence of a stabilizer may not always be obvious from the appearance of a sample.
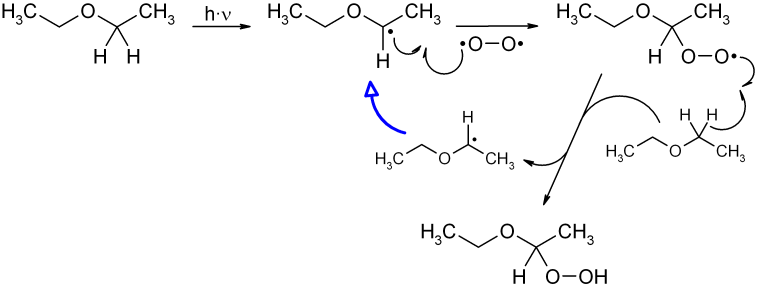
Diethyl ether peroxide removal. There s no information regarding the stopping of formation on the page and frankly my google searches for safe storage of diethyl ether and stop diethyl ether peroxide formation seem to come up with put some iron wire in it or potassium hydroxide. Purchase ethyl ether that contains peroxide inhibitors such as bht or ethanol. Diethyl ether hydroperoxide and its condensation products are blamed for the explosive organic peroxides that slowly form upon exposure of diethyl ether to ambient air and temperature conditions. Present treat the solvent to remove the peroxides.
A sodium lead alloy can also be used but is not recommended as there may be problems with disposing of the lead which is toxic after the reaction is. Diethyl ether ether tetrahydrofuran thf diethylene glycol dimethyl ether diglyme tetralin tetrahydronaphthalene. Call the chemical waste program for packaging and removal. Diethyl ether and tetrahydrofuran are two of the.
Divinyl ether peroxide hazard after prolonged storage. Diethyl ether hydroperoxide can be formed by. This is what scares me. Less than 80 ppm peroxides.
Aluminium oxide can also be used to remove peroxides from solvents as well as for drying the solvent. Isopropyl ether seems unusually susceptible to peroxide and there are reports that a half filled 500 ml bottle of isopropyl ether peroxidized despite being kept over a wad of iron wool. Solution is okay to use. Test for peroxide formation monthly after expiration.
Call the chemical waste program who will contact the bomb squad. Many of the organic solvents commonly used in laboratories have the potential to form explosive peroxide crystals. The polymeric peroxides sometimes. Diethyl ether hydroperoxide is the organic compound with the formula c 2 h 5 och ooh ch 3 it is a colorless distillable liquid.
Dicyclopentadiene diethyl ether ether diethylene glycol dimethyl ether diglyme dioxane p dioxane. Even with less than 30 ppm of peroxide if a very large volume of ether is distilled to dryness then a noticeable level of peroxide could potentially still build up. Peroxide forming chemicals overview peroxide forming chemicals are a class of compounds that have the ability to form shock sensitive explosive peroxide crystals. 30 gm of aluminium oxide is sufficient to remove the peroxide from 250 ml of diethyl ether.
Failure to remove peroxides can result in. The peroxide test detects inorganic and organic compounds containing a peroxide or a hydroperoxide group.