Water And Diethyl Ether Are Immiscible Liquids
Immiscibility is a result of two liquids having different polarity.
Water and diethyl ether are immiscible liquids. If more diethyl ether is added a separate diethyl ether layer will appear floating above the water. Diethyl ether for example is partially miscible with water. Most solvents show some miscibility in one another although it might be very low. The most common pair of extraction solvents used is diethyl ether often referred to as simply ether and water.
Mek methyl tert butyl ether mtbe pentane 1 propanol tetrahydrofuran thf toluene trichloroethylene water xylene. C 6 h 11 cooh has a p k a of 4 8 and of 10 7. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. Immiscible miscible solvent miscibility chart.
It was formerly used as a general anesthetic until. Charged compounds dissolve in water and uncharged compounds dissolve in ether. Immiscible liquids do not dissolve in each other. Water and diethyl ether are immiscible liquids.
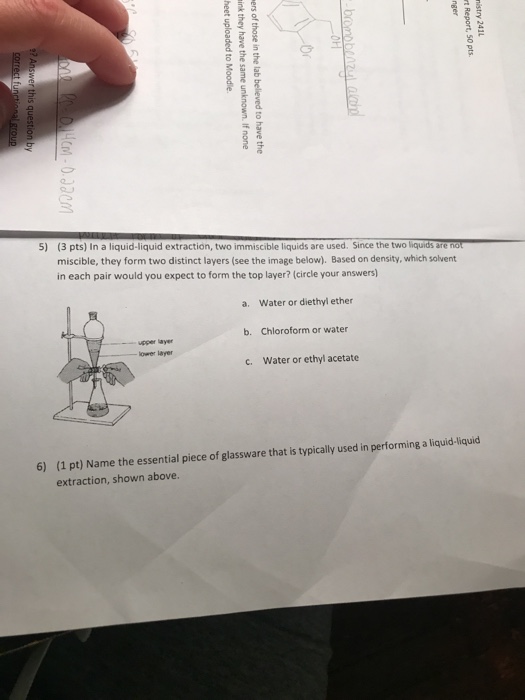
Polarity is a relative term ether is considered nonpolar. Dichloromethane diethyl ether. Charged compounds dissolve in water and uncharged compounds dissolve in ether. What ph would you make the water layer in order to cause both compounds to dissolve in it.
Water and diethyl ether are immiscible liquids. Charged compounds dissolve in water and uncharged compounds dissolve in ether section 3 7. They form layers when placed in the same glassware.