Trimyristin Extraction Diethyl Ether
The extraction of trimyristin can also be done with diethyl ether at room.
Trimyristin extraction diethyl ether. The microwave uses a closed system rationalize why the microwave extraction required only 7 minutes while the conventional extraction required 45 minutes. The purpose of this experiment was extract isolate and purify the natural product trimyristin from the spice nutmeg. Get the latest public health information from cdc. Trimyristin extraction using four other solvents methanol dichloromethane acetone and cyclohexane in addition to diethyl ether.
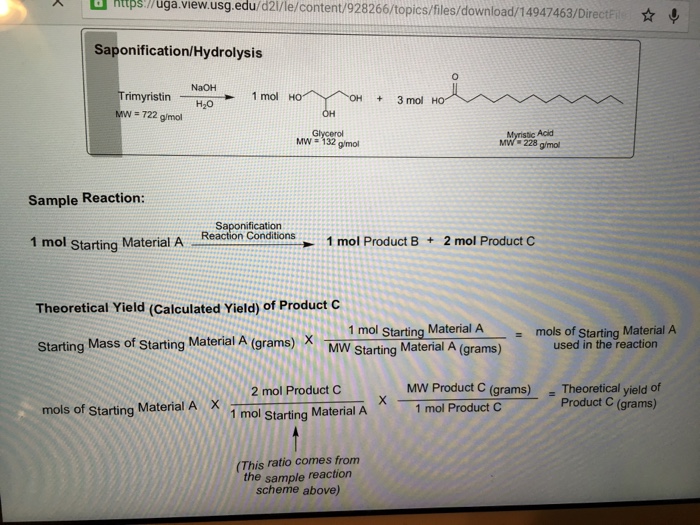
Trimyristin makes up between 20 25 of the overall mass of dried ground nutmeg. However acetone is an organic solvent that can more easily dissolve trimyristin at a higher temperature than diethyl ether. Trimyristin makes up between 20 25 of the overall mass of dried ground nutmeg. The extraction of trimyristin can also be done with diethyl ether at room.
Personalized courses with or without credits. Tripalmitin the structure of which you should look up in a reference source has mp 66 67 oc. Get the detailed answer. Separation is generally effected by steam distillation and purification uses extraction from ether followed by distillation or rotary evaporation to remove the volatile solvent.
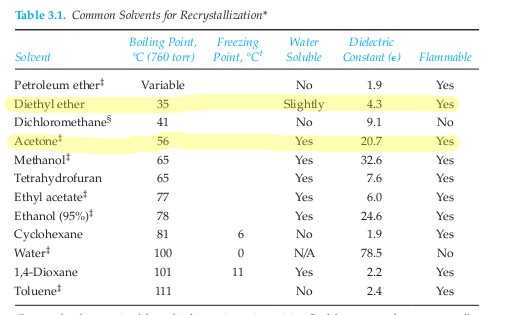
Trimyristin c45h86o6 cid 11148 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information supplier lists and more. Extraction of these two compounds from the plant material with diethyl ether gave. Rank these five solvents from least to most polar citing the basis for your ranking. Diethyl ether is a relatively organic substance that can easily dissolve trimyristin more so than aqueous solvent.
Once the oil was extracted the trimyristin was crystallized from the oil by using acetone. What is the boiling point of diethyl ether. The extraction of trimyristin can also be done with diethyl ether at room temperature. Trimyristin comprises approximately 30 of the mass of nutmeg.
Separation is generally carried out by steam distillation and purification uses extraction from ether followed by distillation or rotary evaporation to remove the volatile solvent. The trimyristin was extracted by using the solvent diethyl ether and heat to draw the organic oil out of the ground nutmeg. Trimyristin makes up between 20 25 of the overall mass of dried ground nutmeg. Your dashboard and recommendations.
Trimyristin is more soluble in ether than in acetone. 5 a certain plant material is known to contain mainly trimyristin and tripalmitin in approximately equal amounts. Reflux à obtain a heating mantle 250ml beaker water buret clamp retort stand. Based on polarity considerations consider how effectively these solvents will extract timyristin from nutmeg.
How is it possible to surpass the boiling point. Separation is generally carried out by steam distillation and purification uses extraction from ether followed by distillation or rotary evaporation to remove the volatile solvent.