Methyl Ethyl Ether Polar Or Nonpolar

This henry s law constant indicates that ethyl methyl ether is expected to volatilize from water surfaces 2 src.
Methyl ethyl ether polar or nonpolar. For many purposes yes. Could you please help for the tips i would like to know whether ethyl ether or other name diethyl ether is the non polar is because i am interested to use it for extraction of wet algae. Chcl3 chloroform or trichloromethane polar. Based on this henry s law constant the estimated volatilization half life from a model.
Used as a solvent and to make other chemicals. Is ethyl acetate polar or nonpolar. 1 39e 003 mean vp of antoine. Ch4o methanol polar.
Ethyl acetate hexane 0 30 most popular combination sometimes tough to remove solvents completely on rotary evaporator ether pentane 0 40 very popular easy to remove on the rotary evaporator. The ester functional group has a similar character to the ketone and aldehyde functional group. Methyl ethanoate 7 ester. Ethyl acetate is polar.
By bagus amin januari 23 2018 add comment. It is extremely flammable and its inhalation may cause asphyxiation or dizziness. Predicted data is generated using the us environmental protection agency s episuite. Burdick jackson solvents are arranged in order of increasing polarity index a relative measure of the degree of interaction of the solvent with various polar test solutes.
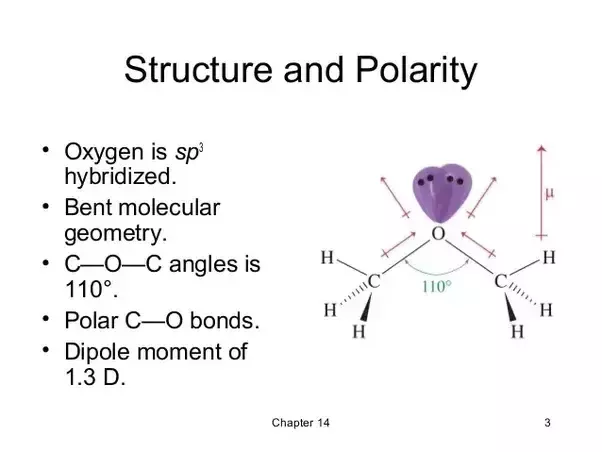
The boiling point indicates that it is the least polar of the three. Methoxyethane also known as ethyl methyl ether is a colorless gaseous ether with a medicine like odor. 114 49 mean or weighted mp vp mm hg 25 deg c. Cyclohexane petroleum ether hexane pentane.
Methyl propyl ether appears as a colorless volatile liquid with an ether like odor. Less dense than water and insoluble in water. Vapors heavier than air. May be narcotic by inhalation in high concentrations.
20 62 adapted stein brown method melting pt deg c. The henry s law constant for ethyl methyl ether is estimated as 6 7x10 4 atm cu m mole src using a fragment constant estimation method 1. Ethers are considered somewhat polar aprotic solvents because while they cannot donate hydrogen bonds they can accept them and also are able to solvate cations but not anions. Dichloromethane diethyl ether toluene.
Ch3och3 dimethyl ether polar. Ch3oh methanol or methyl alcohol polar. Boiling pt deg c. Log octanol water partition coef src.