Is Water Or Diethyl Ether More Polar Solvent
By changing the ph of the solution from neutral to basic the polarity of the benzoic acid changes from nonpolar to polar and thus this change in polarity.
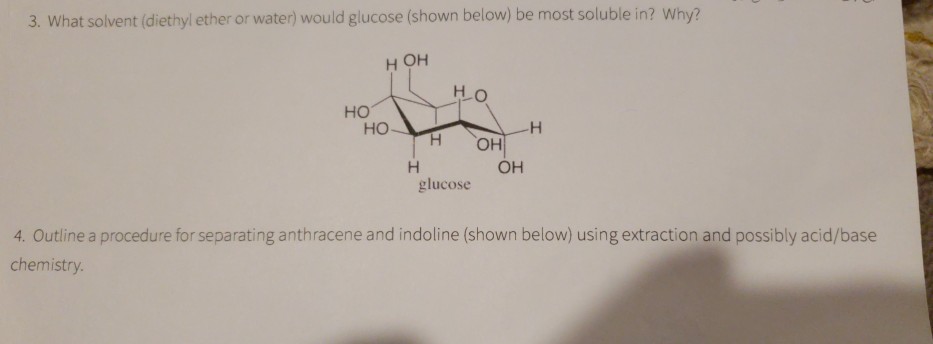
Is water or diethyl ether more polar solvent. Diethyl ether is c2h5 oc2h5 that is a link of c o c. Water is more polar than diethyl ether and this is because the electronegative difference between oxygen and hydrogen is greater than the electronegative difference of carbon and oxygen thus making water the more polar substance. But this is a matter of degree. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines.
Question is diethyl ether c2h5 2o or ch3ch2och2ch3 polar or nonpolar. Oxygen is more electronegative than h hence attract pair of electrons in the bond. Could you please help for the tips i would like to know whether ethyl ether or other name diethyl ether is the non polar is because i am interested to use it for extraction of wet algae. That is why water is polar.
Polar in chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Electro negativity difference between carbon and oxygen is less hence less attraction of pair of electrons in the c o bond. How many grams of sodium hydroxide are required to make 1 liter of a 3m solution of naoh. It was formerly used as a general anesthetic until.
Answer diethyl ether c2h5 2o or ch3ch2och2ch3 is nonpolar what is polar and non polar. O becomes slightly negative and h slightly positive. Why is water a more polar solvent than diethyl ether.