Is Diethyl Ether More Soluble In Water Or Hexane

Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines.
Is diethyl ether more soluble in water or hexane. Diethyl ether is more commonly known as just ether. It was formerly used as a general anesthetic until. Ethanol ch3ch2oh is more soluble in diethyl ether ch3ch2och2ch3 than in hexane c6h14. Nonpolar compounds are generally more soluble in diethyl ether than in alcohols such as ethanol because ethers do not have a hydrogen bonding network that would have to be broken up to dissolve the solute.
The reason is because water is more polar than ether and can hydrogen bond with water more freely than with ether since water has both hydrogen bond donor and acceptor atoms. Glucose for one is actually more soluble in water than it is in ether. Di ethyl ether is more polar than n hexane. Pet ether diethyl ether ethyl acetate chloroform acetone n butanol methanol and water.
Ethers such as diethyl ether are good solvents for a wide range of polar and nonpolar organic compounds. It also has nonpolar side chains which are not attracted to the polar molecule water. However not all of the compounds you listed are more soluble in diethyl ether than water. Diethyl ether is soluble with water to some extent 6 9g 100ml.
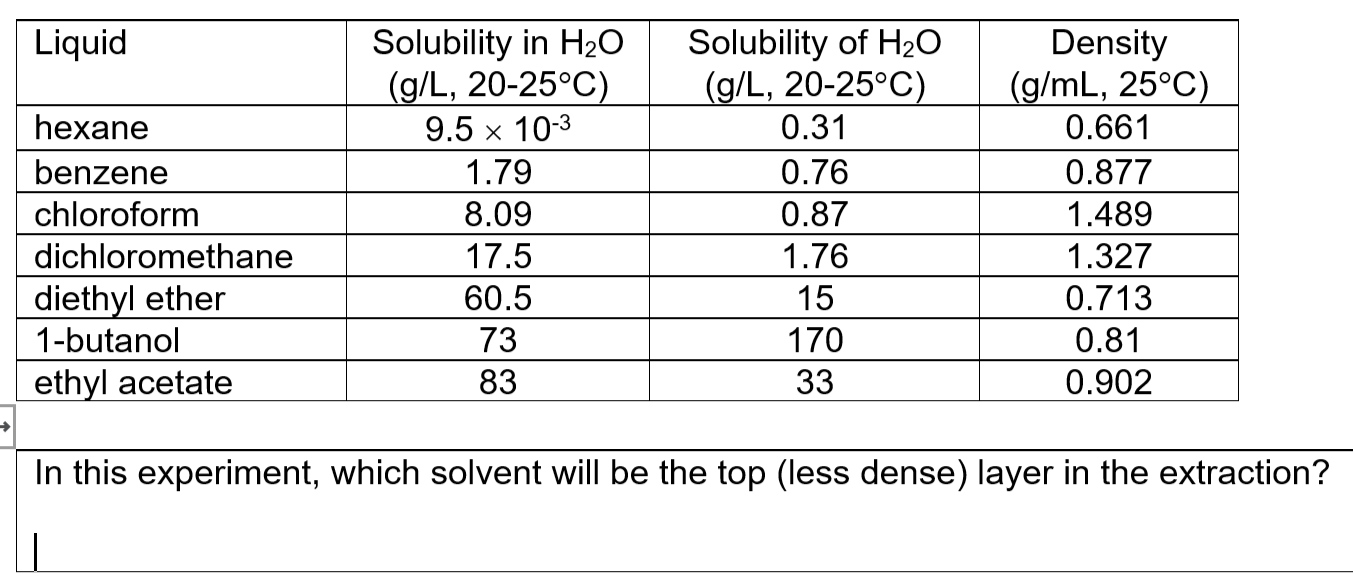
The density of diethyl ether is 0 7134 grams per cubic centimeter.