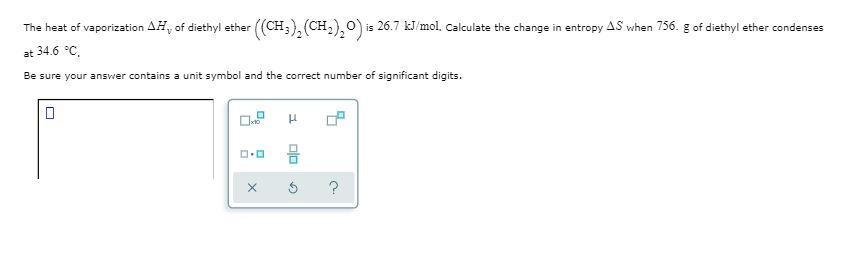
Heat Of Vaporization Of Diethyl Ether
Degree c the heat of vaporization of diethyl ether would be expected to be than the heat of vaporization of carbon disulfide.
Heat of vaporization of diethyl ether. The specific heat and heat of vaporization of liquid ethyl ether at 0 and 12 j. Heat capacity c p. As an example of using the clausius clapeyron equation given that the vapor pressure of benzene is 1 atm at 353 k and 2 atm at 377 k benzene s heat of vaporization is obtained as 32 390 j mol within that temperature range. Ether diethyl ethane 1 1 oxybis.
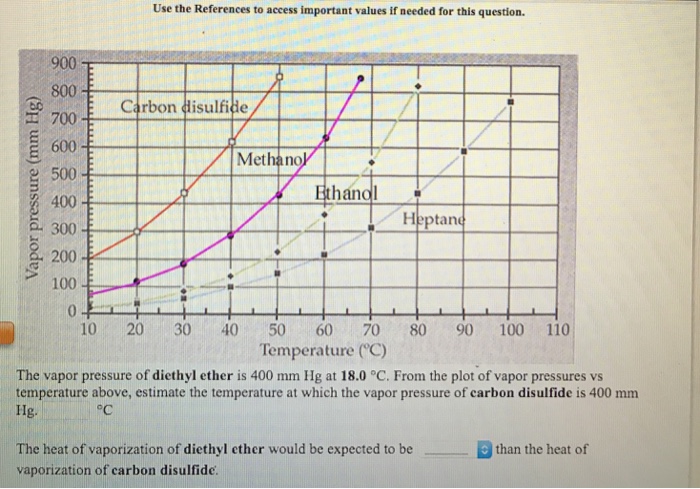
The experimental data shown in these pages are freely available and have been published already in the ddb explorer edition the data represent a small sub list of all available data in the dortmund data bank for more data or any further information please search the ddb or contact ddbst. Heat capacity at saturation pressure heat capacity at saturation pressure liquid in equilibrium with gas as a function of temperature temperature from 149 874 k to 457 413 k. Chemical and physical properties of dimethyl ether. From the plot of vapor pressures vs temperature above estimate the temperature at which the vapor pressure of carbon disulfide is 400 mm hg.
The density of gaseous dimethyl ether j. Latent heat of vaporization of fluids alcohol ether nitrogen water and more sponsored links the input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. Stephenson and malanowski 1987. The enthalpy of vaporization molar heat of vaporization at 298 k for diethylether c4h10o is 26 0 kj mol.
Soc 1941 63 2267 2272. Structure and properties. Stephenson and malanowski 1987. Enthalpy of vaporization or sublimation liquid to gas as a function of temperature.
Heat of vaporization of diethyl ether. Chemical properties of dimethyl ether cas 115 10 6 download. Enthalpy of vaporization at standard conditions kj mol. The vapor pressure of diethyl ether is 400 mm hg at 18 0 degree c.
How much heat would be required to vaporize 1 00 l of the ether at 298 k if its density is 0 714 g ml. Soc 1924 46 1753 1760. The heat capacity and entropy heats of fusion and vaporization and the vapor pressure of dimethyl ether. The heat of vaporization is constant over the temperature range as defined by t 1 and t 2.
A calorimeter for measuring specific heats and heats of vaporization of liquids.