Diethyl Ether And Hexane Miscible
Now place an ethyl group on the second hexane and the fourth.
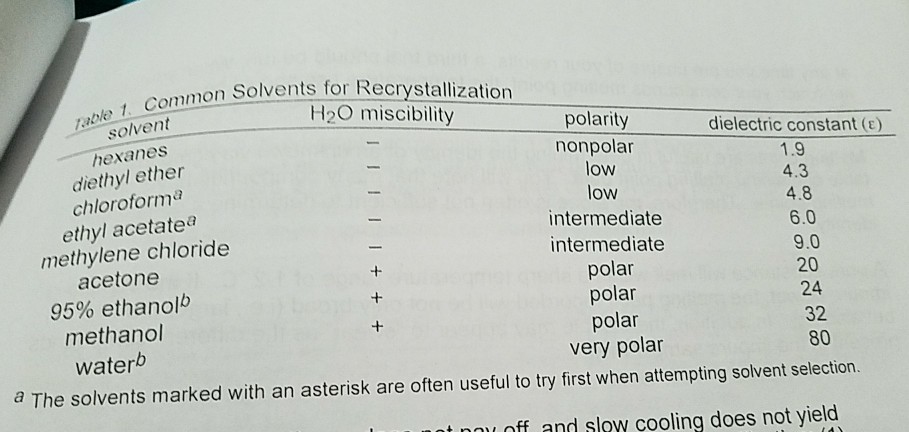
Diethyl ether and hexane miscible. Miscible means the substances mix completely. For example tetrahydrofuran thf and water are miscible. R o r ethers diethyl ether r x alkyl halides tetrachloromethane chloroform r coor esters ethyl acetate r co r aldehydes acetone methyl ethyl and ketones ketone r nh 2 amines pyridine triethylamine r oh alcohols methanol ethanol isopropanol butanol r cohn 2 amides dimethylformamide r cooh carboxylic acids ethanoic acid. Many solutions used in separatory funnels are fairly dilute so the density of the solution is approximately the same as the density of the solvent.
For example if mixing diethyl ether and a 10. Finally for part d we worked with the solubility of organic acids and bases. The four compounds that we used were ethyl alcohol diethyl ether methylene chloride and hexane. Same principles from part b apply here.
Especially good for weight control. To draw 2 4 diethyl 4 ethoxyhexane start by drawing hexane. When substances are miscible there are no layering effects precipitates partial mixing or separation. Also place an o atom.
While on the other hand nonpolar substances like diethyl ether were not miscible mix with polar h2o. Solvent miscibility table iso octane n hexane n heptane di ethyl ether cyclohexane ethyl acetate toluene chloroform tetrahydrofuran benzene acetone dichloromethane dioxane n propanol ethanol dimethylformamide acetonitrile acetic acid dimethyl sulfoxide methanol water iso octane n hexane n heptane di ethyl ether cyclohexane ethyl acetate toluene. Ce naoh left aq right solution in a separatory funnel knowledge of the exact density of the 10. If two substances are miscible they are also completely soluble in one another irrespective of the order of introduction.
Acetic acid acetone acetonitrile benzene butanol butyl acetate n carbon tetrachloride chloroform cyclohexane dichloroethane 1 2 dichloromethane diethyl ether diisopropyl ether dimethylformamide dimethyl sulfoxide dmso dioxane ethanol ethyl acetate heptane hexane isooctane isopropanol methanol methyl ethyl ketone. Ce naoh solution is not. Polar substances like ethyl alcohol and h2o were miscible. This is the same case for diethyl ether and methylene chloride and diethyl ether and ethyl alcohol.