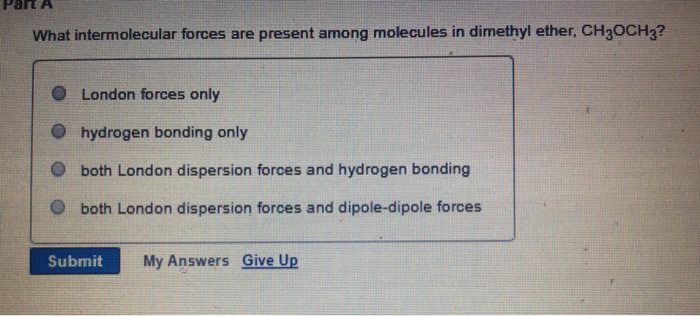
Does Diethyl Ether Have Dipole Dipole Forces
C4h10o diethyl ether oxygen carbon hydrogen unshared electrons when c4h10o interacts with c4h10o there are two intermolecular forces that occur.
Does diethyl ether have dipole dipole forces. H h h h. The h of c4h10 is attracted to the o of h2o. Diethyl ether is somewhat polar because of the two unbonded electron pairs around the oxygen atom. Diethyl ether this one experiences temporary dipole induced dipole td id interactions c h and c c and permanent dipole dipole interaction c o.
H c c o c c h. Both molecules are the same. The positive h of c4h10o is attracted to the negative o of c4h10o. Does diethyl ether had dipole dipole forces.
So for the temporary dipole induced dipole interaction it is a non polar homonuclear molecule. These two molecules are adjacent in a liquid so they are attracted by dispersion forces. The positive h of h2o is attracted to the negative o of c4h10o.