Is Diethyl Ether A Polar Molecule
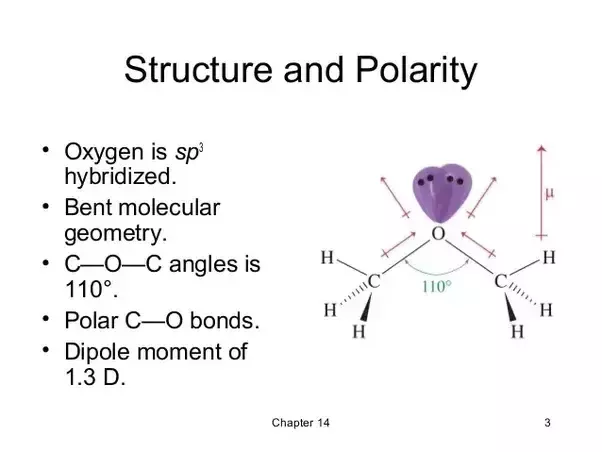
It was formerly used as a general anesthetic until.
Is diethyl ether a polar molecule. Diethyl ether being a symmetrical molecule theb dipole moments directed toward the ethrial o atom cancel out with each other making the molecule non polar almost thf on the other hand is not non. Thus basic substances such as grignard reagents can be. Because diethyl ether has a dipole moment polar substances readily dissolve in it. Ethers such as diethyl ether dissolve a wide variety of organic compounds of polar and non polar origin.
It does not have different charges and chemical reactions at different points along its structure so it is a non polar organic compound. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. Polar in chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. The boiling point indicates that it is the least polar of the three.
Diethyl ether is extremely flammable and might be explosive. It is a relatively polar molecule and it can form hydrogen bonds with water. In diethyl ether non polar compounds are usually more soluble than alcohols because ethers do not have a hydrogen bonding network that needs to be broken up to dissolve the solute. With reference to the structures and specific functional group s of each molecule why is cholesterol more soluble in acetone than lecithin view the full answer.
The carbon oxygen carbon bond in ethers is much like the carbon carbon bond in alkanes. Answer diethyl ether c2h5 2o or ch3ch2och2ch3 is nonpolar what is polar and non polar. 3 acetone is a more polar solvent than diethyl ether. Polar compounds that can serve as hydrogen bond donors dissolve in diethyl ether because they can form hydrogen bonds to the nonbonding electron pairs of the ether oxygen atoms.