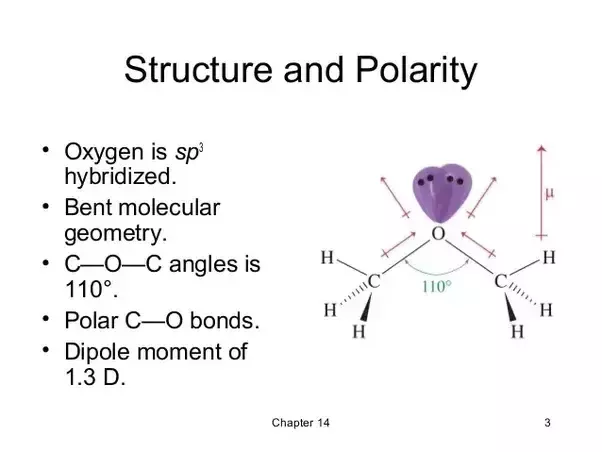
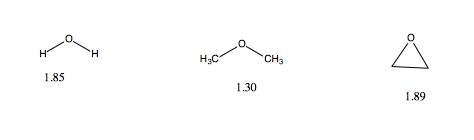
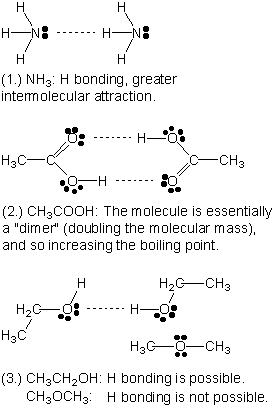
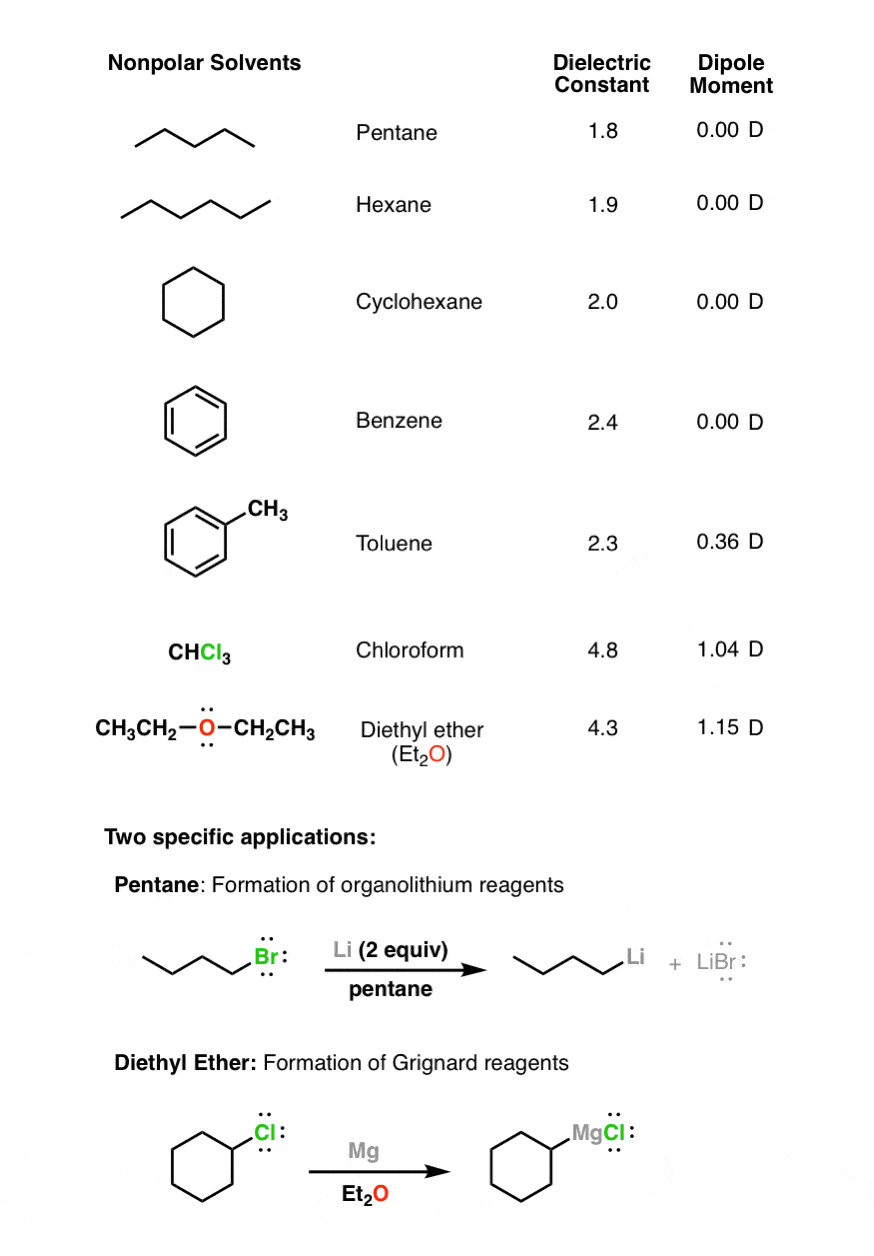
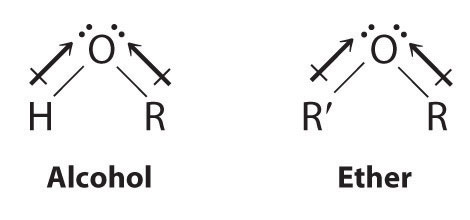
Dimethyl Ether Dipole Moment
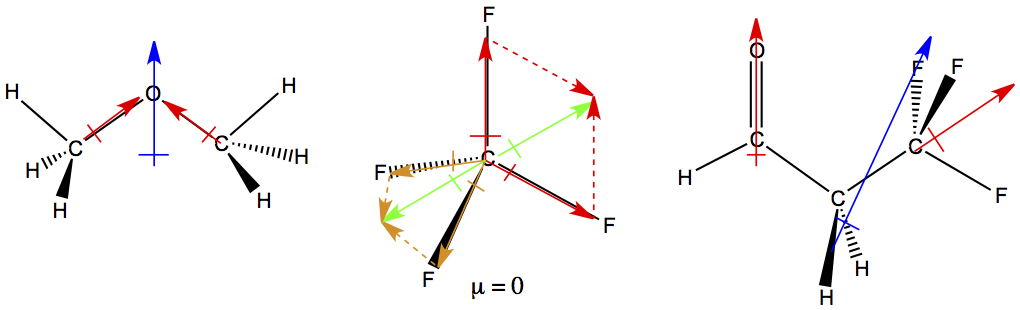
Dipole moment of diethyl ether.
Dimethyl ether dipole moment. The conformers are designated as gauche trans axial etc. In some cases an average value obtained from measurements on the bulk gas is. From the dependence of the moments of these compounds on the number of oxygen atoms as well as from the temperature dependence of the moments of monoxymethylene dimethyl ether it was concluded that these compounds are mixtures of rotational isomers the predominant ones of. The simplest ether it is a colorless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applications it is an isomer of ethanol.
The dipole moments of monoxymethylene dimethyl ether and dioxymethylene dimethyl ether were measured in hexane solution. Dipole moments of individual conformers rotational isomers are given when they have been measured. Bond dipole moments are derived for the o h c h and c o bonds from ab initio calculated and experimentally determined electrostatic multipoles of dimethyl ether methanol methane and water using an electrostatic model with point dipoles placed at the midpoints of the bonds between atoms and directed along the bonds. Dimethyl ether dme also known as methoxymethane is the organic compound with the formula ch 3 och 3 simplified to c 2 h 6 o.
The experimental data shown in these pages are freely available and have been published already in the ddb explorer edition the data represent a small sub list of all available data in the dortmund data bank for more data or any further information please search the ddb or contact ddbst. Dipole moment this page provides a list of dipole moment for about 800 molecules including organic and inorganic.