Diethyl Ether Water Reaction

Because of its characteristics diethyl ether was widely used in many countries as an anesthetic agent but was then replaced by other substances in the 1960s.
Diethyl ether water reaction. Chem student 2 1. Diethyl ether inhibits alcohol dehydrogenase and thus slows down the metabolism of ethanol. The latter is predominantly applied for low water concentrations 0 1. Diethyl ether is a common laboratory solvent.
It was formerly used as a general anesthetic until. The main route of exposure is inhalation. A nostalgic neil uses diethyl ether and bubbling nitrogen gas to freeze some water outside a test tube. One exception are the vinyl ethers which cause an interfering side reaction with methanolic karl fischer reagents.
C 2 h 5 oc 2 h 5 6o 2 4co 2 5h 2 o. Halogenation ether reacts with halogens like chlorine or bromine forming halo substituted ether undergoes substitution reaction in the absence of sunlight. Diethyl ether cas 60 29 7 is a component of starting fluids and is used as a solvent in the manufacture of synthetic dyes and plastics. Sciencegoals more sciencegoals videos at.
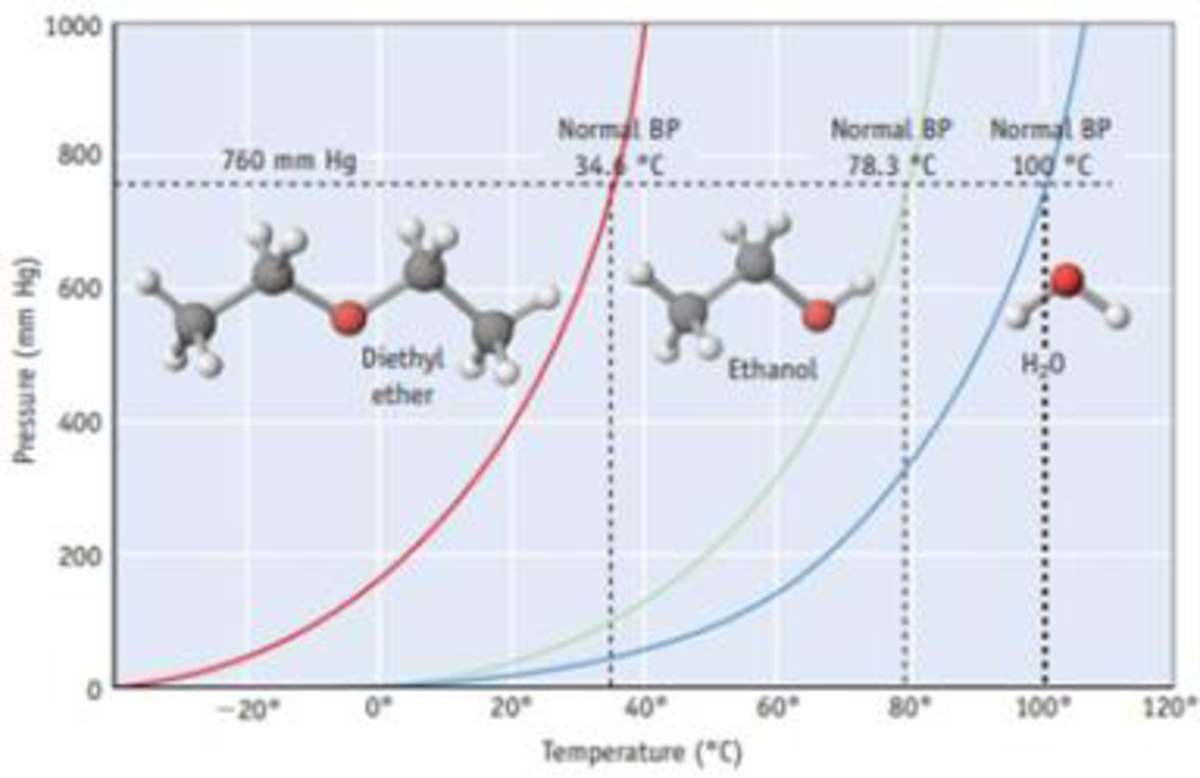
Diethyl ether vapors hug the ground and in dry air explosive peroxides can form. It has limited solubility in water thus it is commonly used for liquid liquid extraction. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. Professional chemists will be well appraised of the hazards presented in using ether but the layperson is less likely to be aware of these dangers.
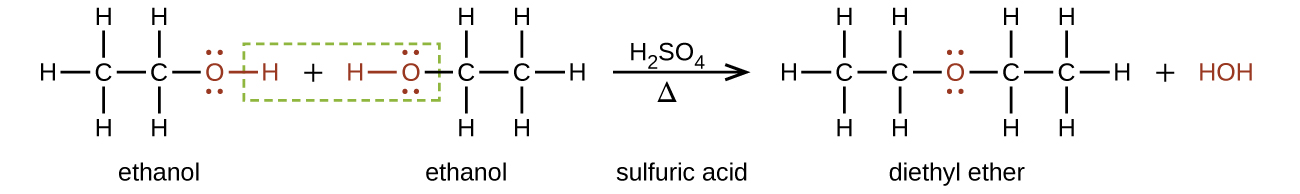
Diethyl ether would be on top because it is non polar so it doesn t mix well with water and because it s less dense so it will float on top of water source s. Diethyl ether or ch 3 ch 2 o ch 2 ch 3 is a great solvent for many things but is extremely flammable. You got it all wrong. Then you said that water is then released and the ethyl alcohol conjugate base is formed ce h3c ch2.
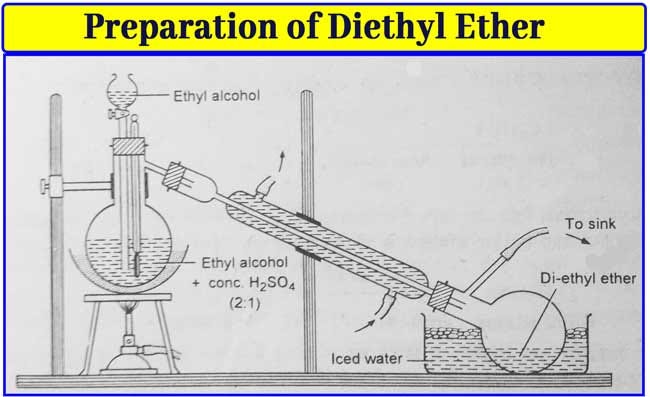
This is the point where you got it. Our thanks to the google making science team. First of all the reaction written by you concisely is ce diethyl ether h2so4 ch3 ch2 oh ch3 ch2 hso4 your first step is correct that oxygen gets protonated due to acid. Being less dense than water.
Recommended methods are both the volumetric titration with one or two component reagents as well as the coulometric analysis.