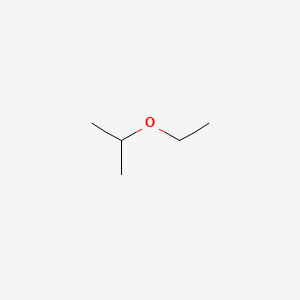
Diethyl Ether Polarity Value

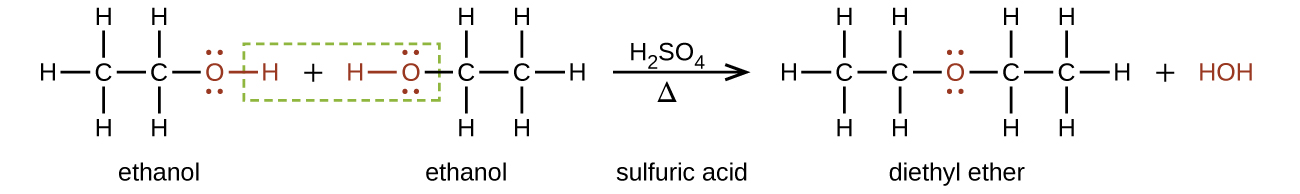
When utilizing extraction solvents for liquid liquid extraction two solvents must be used.
Diethyl ether polarity value. Combustion ether is highly flammable liquid and undergoes combustion reaction resulting in the formation of carbon dioxide and water. All answers 6 11th oct 2017. C 2 h 5 oc 2 h 5 6o 2 4co 2 5h 2 o. It was formerly used as a general anesthetic until.
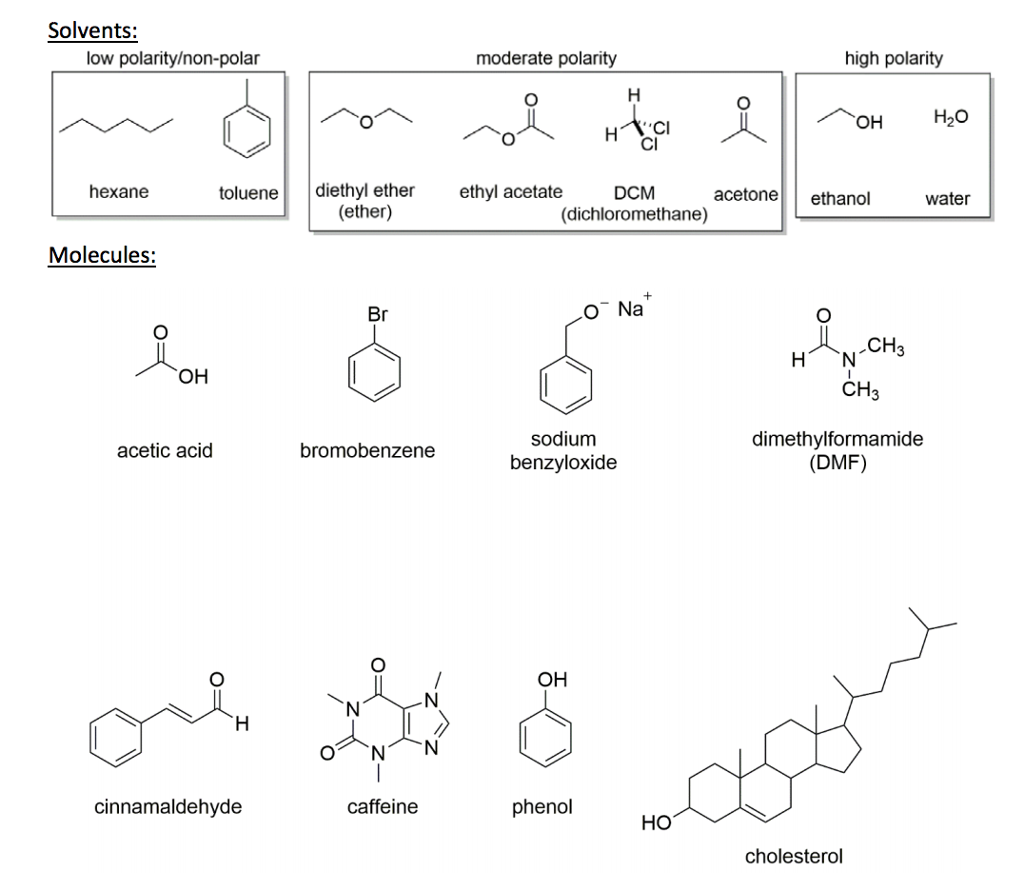
Ether c2h5 2o or c4h10o cid 3283 structure chemical names physical and chemical properties classification patents literature biological activities. Properties of organic solvents. The boiling point indicates that it is the least polar of the three. Microsoft word solvent polarities doc.
Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from. Halogenation ether reacts with halogens like chlorine or bromine forming halo substituted ether undergoes substitution reaction in the absence of sunlight. Diethyl ether is considered a good organic extracting solvent because it has a low polarity according to the university of alberta s organic web chem. One is usually water or water based and the other an organic solvent.
Chemical properties of diethyl ether c 2 h 5 2 o. Diethyl ether benzene toluene xylene carbontetrachloride cyclohexane petroleum ether hexane pentane least polar title. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. Dear peter diethyl ether is considered as a non polar solvent due to its low dielectric constant.
Christian reichardt solvents and solvent effects in organic chemistry wiley vch publishers 3rd ed 2003. The values in the table below except as noted have been extracted from online and hardbound compilations.







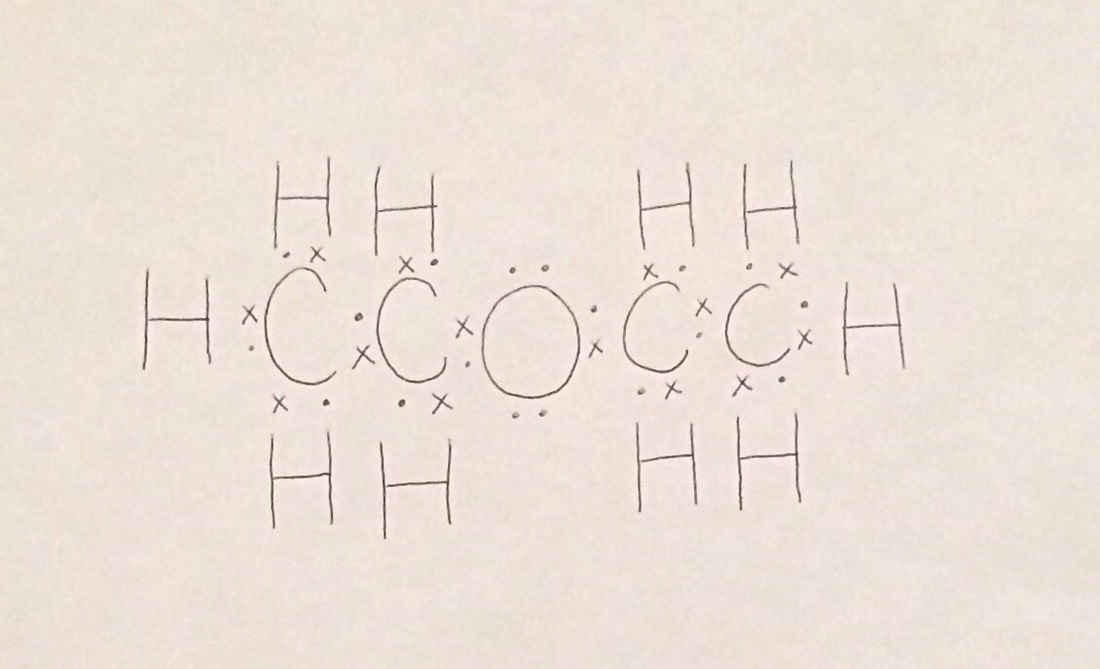

/TLC%202.gif)