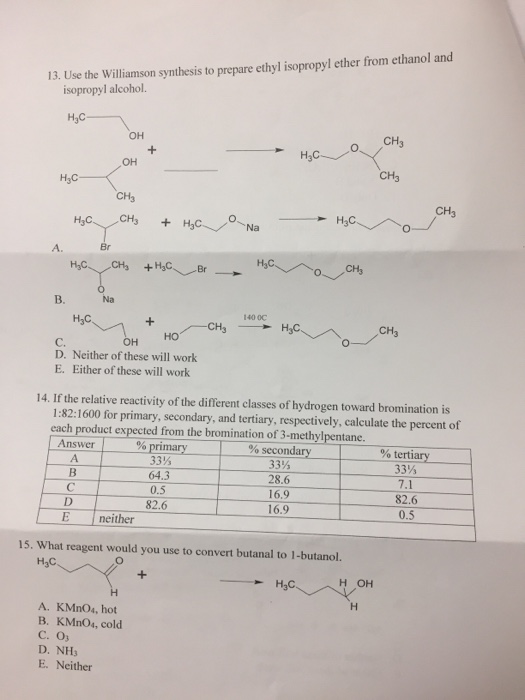
Convert Diethyl Ether To Ethanol
Ethanol was directly synthesized from dimethyl ether dme and syngas with the combined h mordenite and cu zno catalysts that were separately loaded in a dual catalyst bed reactor.
Convert diethyl ether to ethanol. Thereafter ethanol and methanol wer. Diethyl ether ethanol c6h16o2 cid 21585056 structure chemical names physical and chemical properties classification patents literature biological. Methyl acetate ma was formed by dme carbonylation over the h mordenite catalyst. Ethanol or dee in the reaction cell put into contact with a pure powder pressed disk of the catalyst previously activated by outgassing at 773 k.
No significant negative impact of water over diethyl ether. Ethanol vs dimethyl ether. The acid in aqueous medium producing hydronium h3o ion a h protonate the oxygen atom of ethanol and the ethanol molecule giving a positive change. 100g of ethanol dry ethanol is best is placed in a 1 2 liter distilling flask and with good cooling 180g of.
Ethanol conversion and ethylene selectivity increased when the reaction temperatures were raised whereas the diethyl ether decreased. Getting a good yield of ether which in turn requires that ether is distilled out of the reaction mixture before it reverts to ethanol. Ethanol is an alcohol having the chemical formula c 2 h 5 oh. The effect of the presence of water on reaction performance was also evaluated.
In the present work mechanistic pathways and kinetics of catalytic dehydration of ethanol were investigated in a closed batch reactor for the formation of diethyl ether and ethylene over the synthesized nio loaded hzsm 5 in the range of 160 240 c. The reaction to make diethyl ether is reversible so eventually an equilibrium between reactants and products is achieved. The result indicated that dehydration activity significantly depended on the reaction temperature. The melting point of ethanol is 114 1 c.
In this case ethanol is treated by concentrated sulphuric acid h2so4 to form diethyl ether. Dimethyl ether is an ether. Ethanol is an alcohol. After that the oxygen atom of unprotonated ethanol a h2o molecule formed from ethanol and producing diethyl ether.
Dimethyl ether is an ether compound having the chemical formula c 2 h 6 o. This is also called ethyl ether or ether and must not be confused with petroleum ether if for example you see a formula calling for ether then it refers to diethyl ether if it does not specify petroleum ether then do not use petroleum ether. The melting point of dimethyl ether is 141 c. It could be nearly achieved the complete ethanol conversion at 400 c over al ssp composite catalysts.