Why Water And Diethyl Ether Do Not Mix

In your example it s not that some molecules of ether will not dissolves in water and vice versa it s just that if the two liquids are in contact the water will stay with water and the ether with ether for the most part.
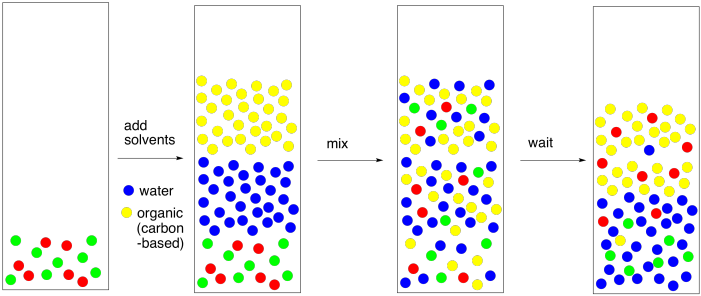
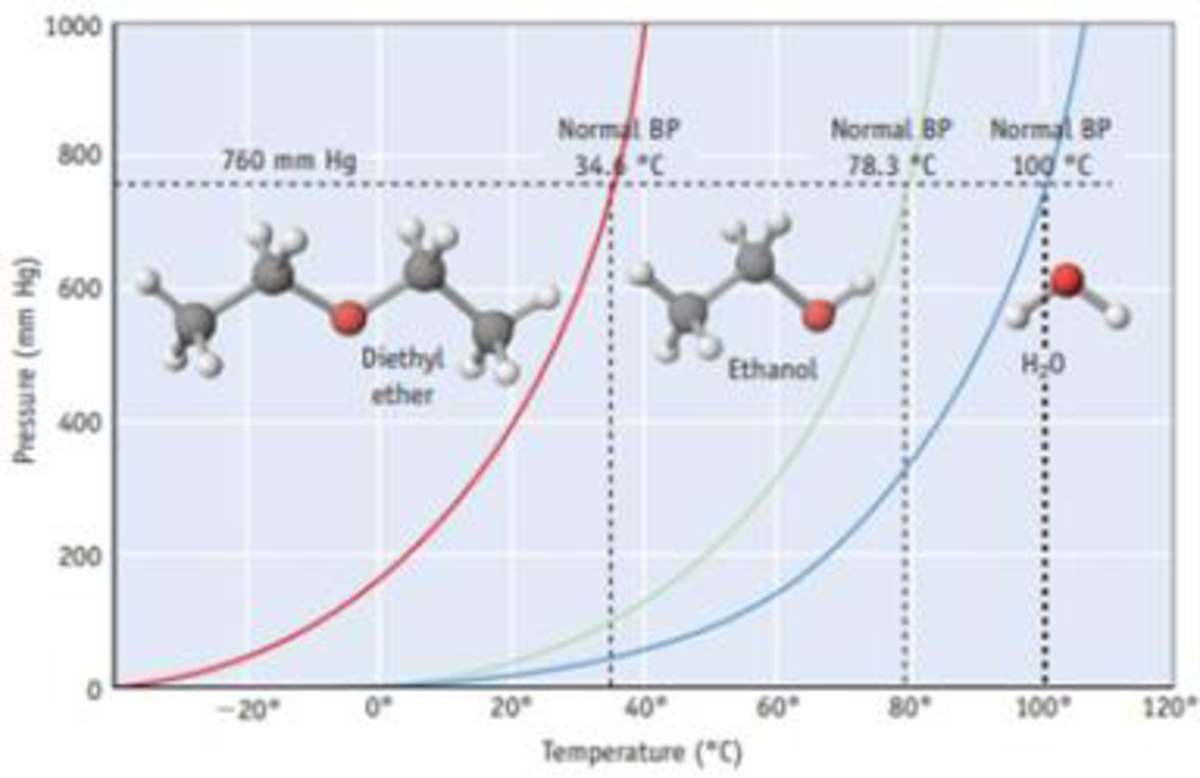
Why water and diethyl ether do not mix. So when you mix hydrocarbons with water they tend to form layers. Diethyl ether cannot participate in hydrogen bonding with water. When two compounds are mutually insoluble they re immiscible with each other. Compounds that are less polar like diethyl ether c4h10o aren t soluble at all in water.
Chem student 2 1. I understand the they can have hydrogen bonding with water and the most of the substances cannot and thus cannot mix. But 1 hexanol c6h14o is only partially soluble in water. It also has nonpolar side chains which are not attracted to the polar molecule water.
It was formerly used as a general anesthetic until. When you mix the polystyrene with solvent the bonds between the chains as well as some internal bonds are broken allowing the gas to escape. It seems to me however that ethyl acetate should be able. Water is not miscible with diethyl ether.
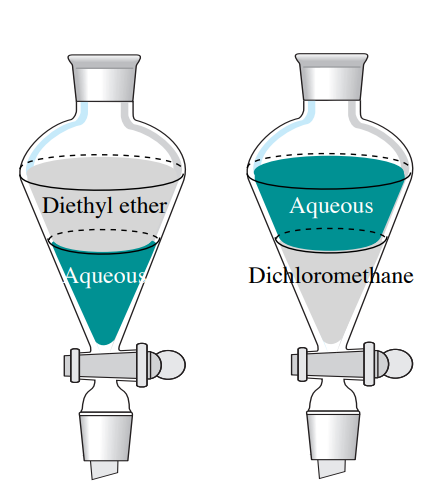
Diethyl ether would be on top because it is non polar so it doesn t mix well with water and because it s less dense so it will float on top of water source s. We did a lab and for part of it we added in separate tubes diethyl ether ethyl acetate dichloromethane acetone ethanol and toluene to water. Diethyl ether is soluble with water to some. The only two that were miscible with water were ethanol and acetone.
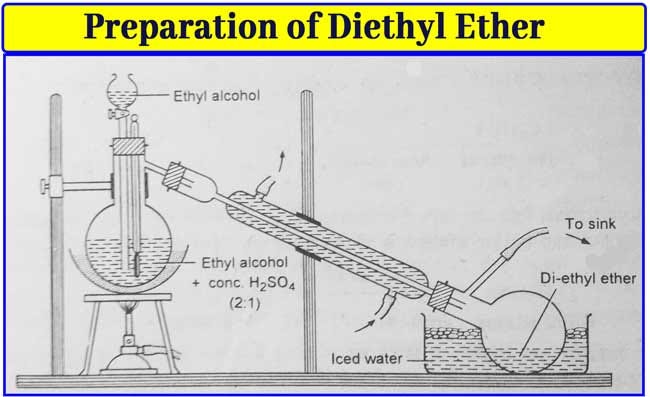
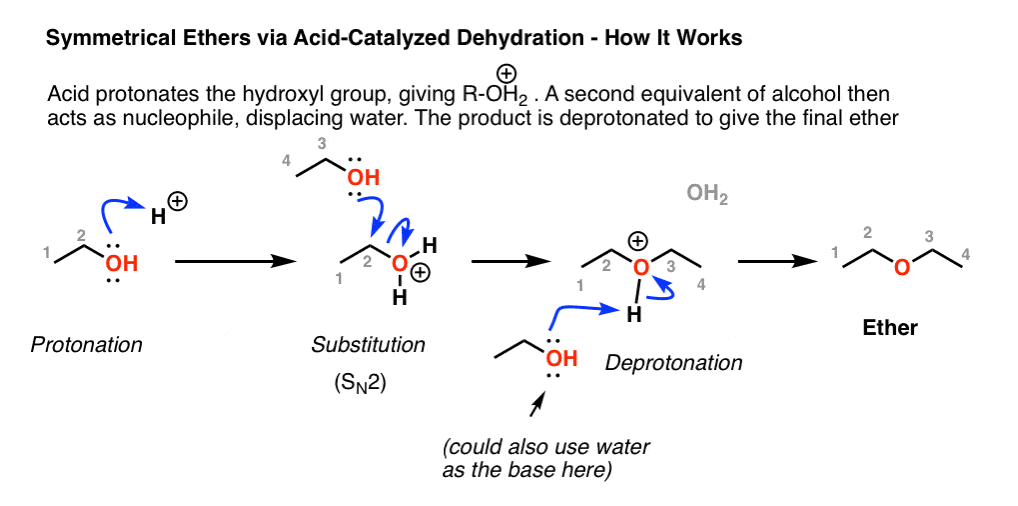
Acetic acid acetone acetonitrile benzene butanol butyl acetate n carbon tetrachloride chloroform cyclohexane dichloroethane 1 2 dichloromethane diethyl ether diisopropyl ether dimethylformamide dimethyl sulfoxide dmso dioxane ethanol ethyl acetate heptane hexane isooctane isopropanol methanol methyl ethyl ketone. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. This is because water s strongest imf is hydrogen bonding while ether s is dispersion with some dipole dipole forces. This simply greatly reduces the volume of the polymer.