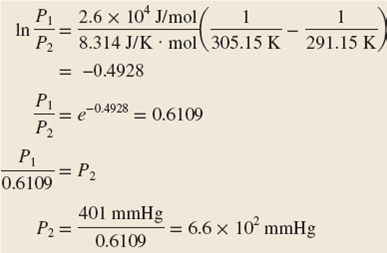What Is Diethyl Ether Vapor Pressure
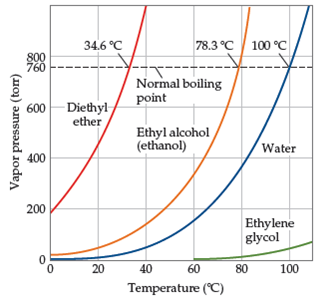
The vapor pressure of a liquid varies with its temperature as the following graph shows for water.
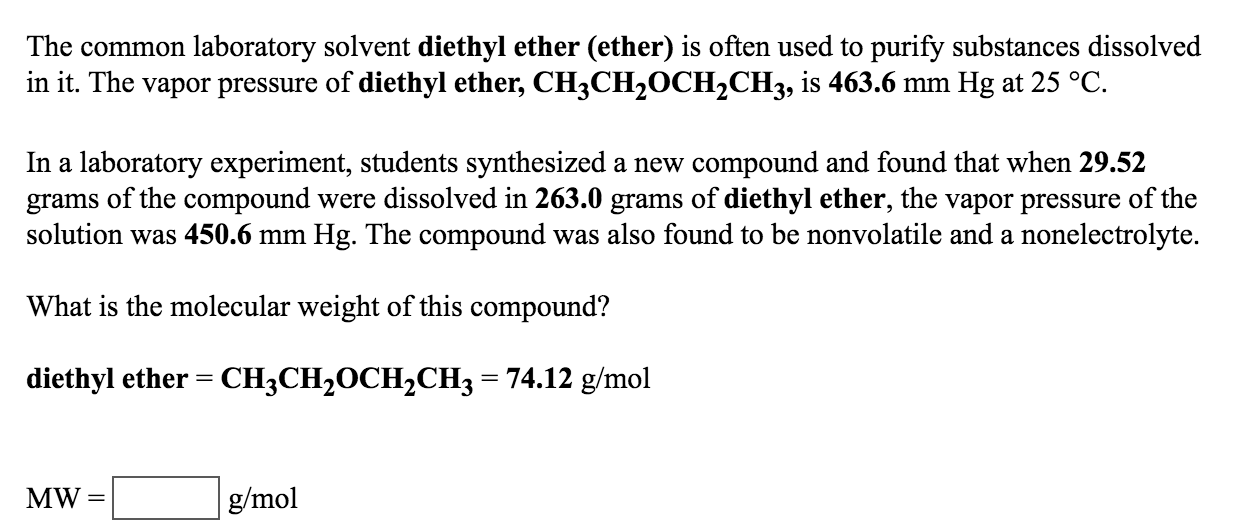
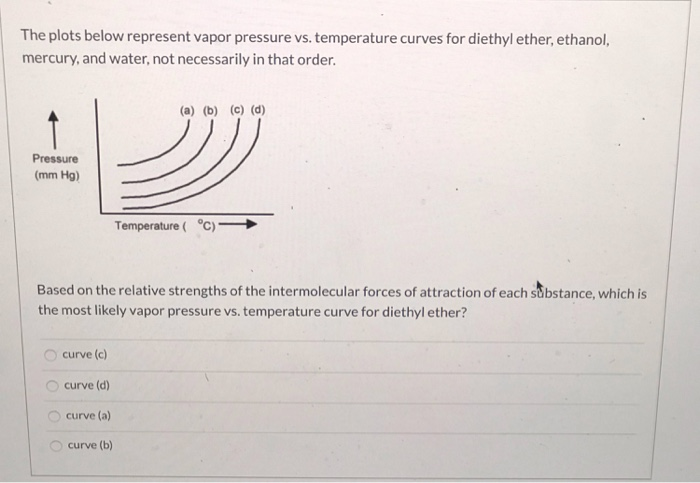
What is diethyl ether vapor pressure. Recall that diethyl ether has weak dispersion forces which meant that the liquid has a high vapor pressure. Vapor pressure of diethyl ether. The volatility of diethyl ether is evident from a vapor pressure more than 20 times that of water at these temperatures. Diethyl ether is a volatile organic compound.
The vapor pressure of dichloromethane at 260 k is 71 torr. It is highly recommended that you seek the material safety datasheet for this chemical from a reliable source such as siri and follow its directions msds for diethyl ether is available at mallinckrodt baker. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. The vapor pressure of water at 20 c is only 2 33 kpa far less than that of diethyl ether.
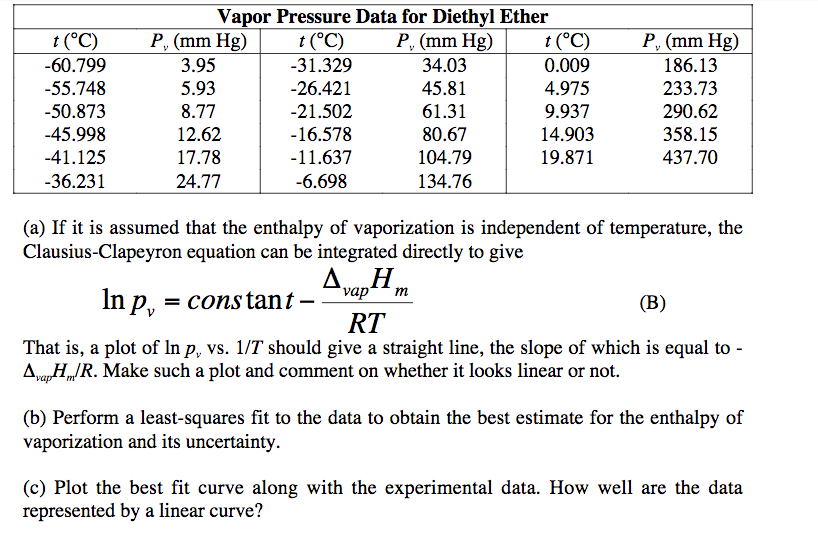
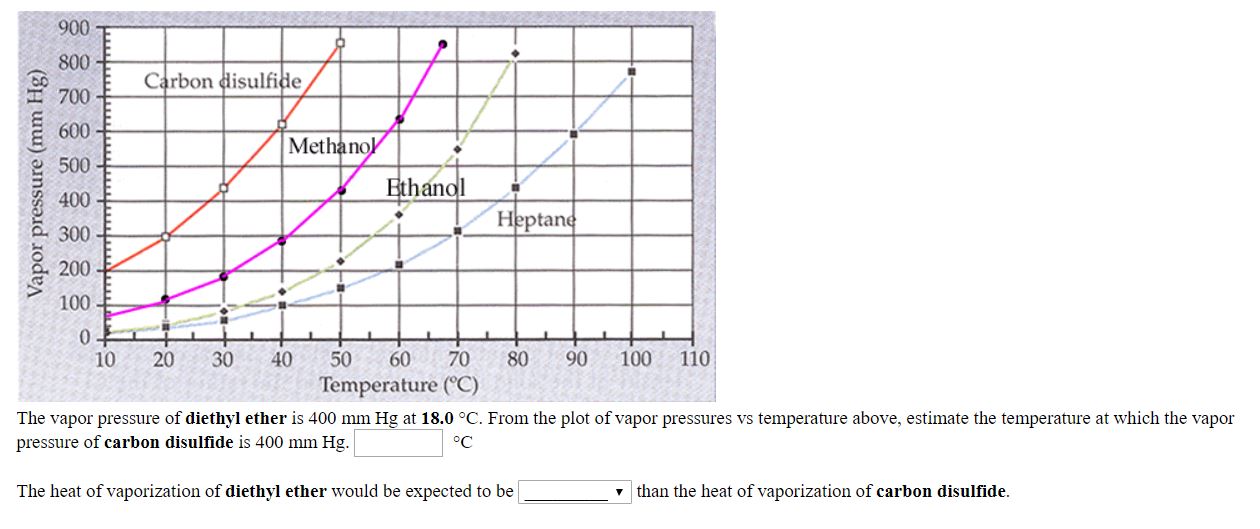
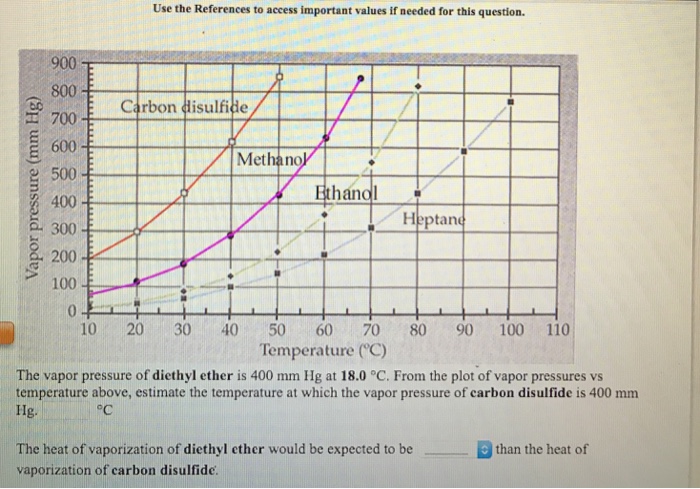
Vapor pressure and temperature. This difference can be demonstrated by means of a buret clamped upside down in a reservoir of water several ml of air trapped in the buret above a column of water and a funnel attached to the buret nozzle. The weak forces also mean that it does not require a large an input of energy to make diethyl ether boil and so it has a relatively low normal boiling point of 34 6 c. The vapor pressure of diethyl ether at 18 degree c is 402 mm hg.
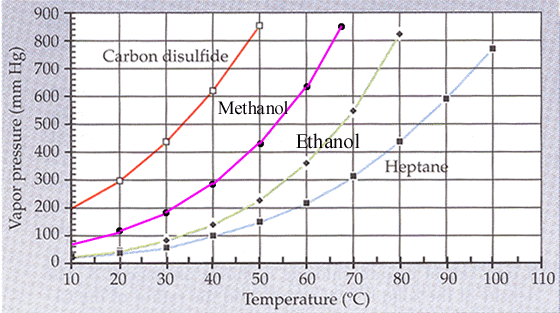
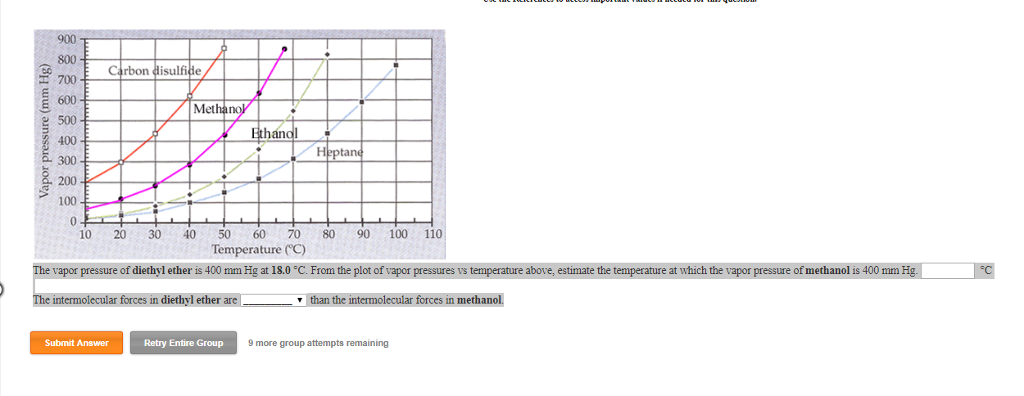
Its vapor pressure at 20 c is 58 96 kpa. Vapor pressure at 25 o c. The vapor pressure of diethyl ether is 401 mm hg at 18c and the hvap 26 0 kj mol. Delta h vap 26 0 kj mol r 8 314 j mol middot k 311 mm hg 781 mm hg 493 mm hg 658 mm hg 164 mm hg the vapor pressure of ethyl alcohol at 34 8 degree c is 100 mm hg.
It was formerly used as a general anesthetic until. Water is a polar liquid whose molecules are attracted to one another by relatively strong hydrogen bonding. How much energy is needed to vaporize 75 0 g of diethyl ether c4h10o at its boiling point 34 6 c given that δhvap of diethyl ether 26 5 kj mol. The line on the graph shows the boiling temperature for water.
Material safety data sheet. What is the vapor pressure at 310 k given that δhvap 31 4 kj mol. What is the new vapor pressure of diethyl ether if the temperature is carefully increased to 32 degree c. The experimental data shown in these pages are freely available and have been published already in the ddb explorer edition the data represent a small sub list of all available data in the dortmund data bank for more data or any further information please search the ddb or contact ddbst.