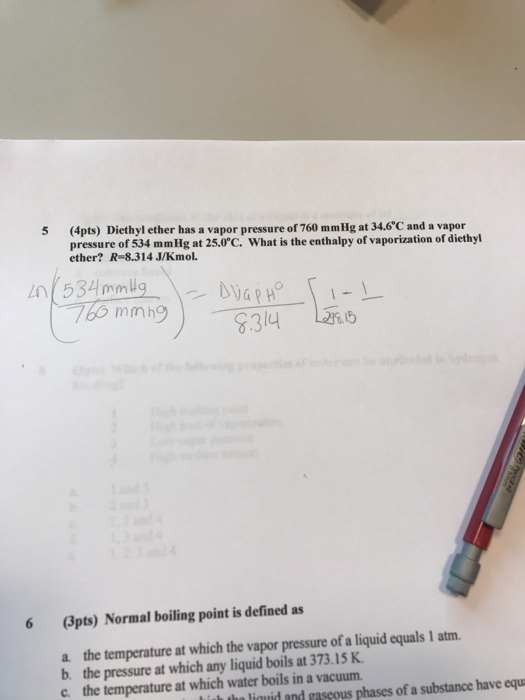The Enthalpy Of Vaporization Of Diethyl Ether
Thus diethyl ether ethyl ether acetone and gasoline are volatile but mercury ethylene glycol and motor oil are nonvolatile.
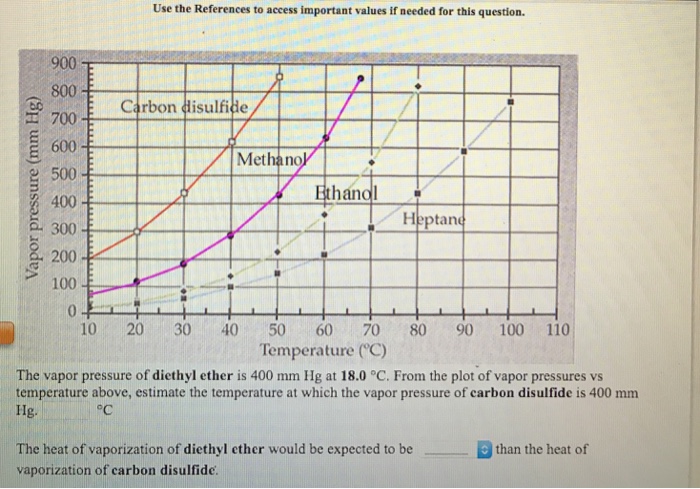
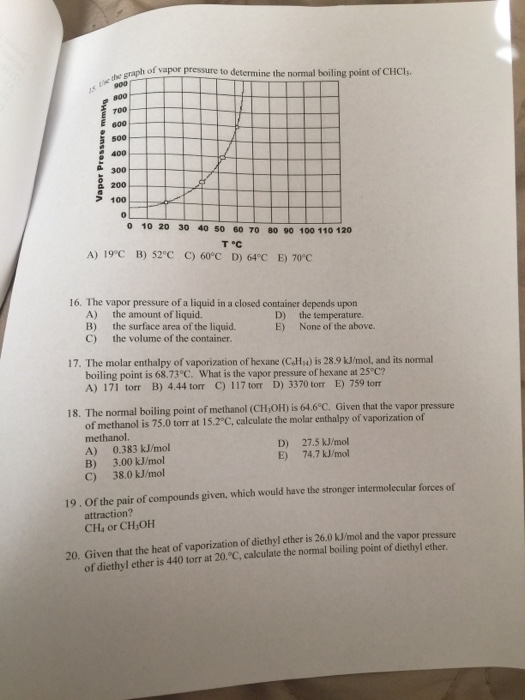
The enthalpy of vaporization of diethyl ether. The enthalpy of vaporization for diethyl ether 26 0 kj mol. The vapor pressures of several liquids as a function of temperature. Soc 1941 63 2267 2272. Calculate δ s o for conversion of.
Ether diethyl ethane 1 1 oxybis. Stephenson and malanowski 1987. A calorimeter for measuring specific heats and heats of vaporization of liquids. The enthalpy of vaporisation of liquid diethyl ether c 2 h 5 2 o is 2 6 k j m o l 1 at its boiling point 3 5 o c.
The density of gaseous dimethyl ether j. Enthalpy of vaporization or sublimation liquid to gas as a function of temperature temperature from 149 874 k to 466 748 k 13 experimental data points heat capacity at saturation pressure. What is the energy change in kj when 0 487 kilograms of diethyl ether c4h10o is condensed from a gas at 34 6 c to a liquid at the same temperature. 1 3497 at 24 8 c abbe number.
The heat capacity and entropy heats of fusion and vaporization and the vapor pressure of dimethyl ether. The specific heat and heat of vaporization of liquid ethyl ether at 0 and 12 j. The experimental data shown in these pages are freely available and have been published already in the ddb explorer edition the data represent a small sub list of all available data in the dortmund data bank for more data or any further information please search the ddb or contact ddbst. Stephenson and malanowski 1987.
The enthalpy of vaporization symbol h vap also known as the latent heat of vaporization or heat of evaporation is the amount of energy that must be added to a liquid substance to transform a quantity of that substance into a gas the enthalpy of vaporization is a function of the pressure at which that transformation takes place. The point at which the vapor pressure curve crosses the p 1 atm line dashed is the normal boiling point of the. Std enthalpy change of vaporization. The enthalpy of vaporization is often quoted for the.
How much heat would be required to vaporize 1 00 l of the ether at 298 k if its density is 0 714 g ml. A liquid to vapour b vapour to liquid at 3 5 o c. Structure and properties index of refraction n d. Msds for diethyl ether is available at mallinckrodt baker.
Soc 1924 46 1753 1760.