Diethyl Ether Vapor Pressure Vs Temperature
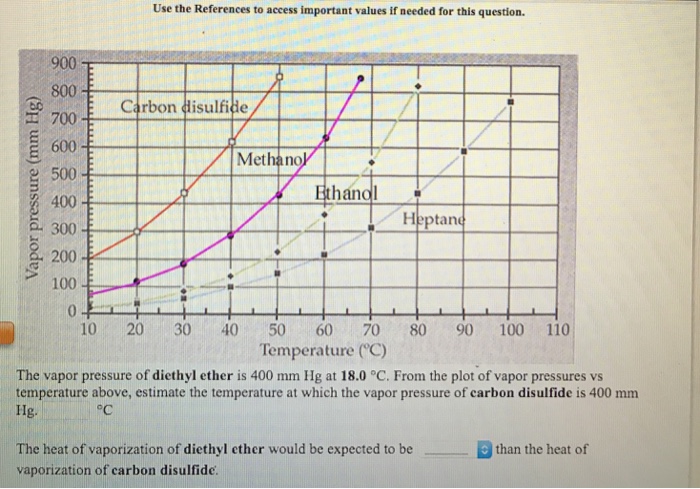
The vapor pressure of diethyl ether is 400 mm hg at 18 0 degree c.
Diethyl ether vapor pressure vs temperature. Log 10 of diethyl ether vapor pressure. Except where noted otherwise data relate to standard ambient temperature and pressure. The vapor pressure of diethyl ether is 400 mm hg at 18 0 c. The diffusion of diethyl ether in air is 9 18 10 6 m 2 s 298 k 101 325 kpa.
Solution for the vapor pressure of diethyl ether is 400 mm hg at 18 0 c. The autoignition temperature of diethyl ether is 160 c 320 f. The pressure required for diethyl ether to boil at 20 c. A common practice in chemical labs is to use steam thus limiting the temperature to 100 c 212 f when ether must be heated or distilled.
Text is available under the creative commons attribution. We can express the nonlinear relationship between vapor pressure and temperature as a linear relationship using the clausius clapeyron equation. From the plot of vapor pressures vs temperature above estimate the temperature at which the vapor pressure of carbon disulfide is 400 mm hg. Vapor pressure at 25 o c.
The vapor pressure of a liquid can be measured in a variety of ways. This page was last edited on 27 november 2019 at 19 21 utc. Degree c the heat of vaporization of diethyl ether would be expected to be than the heat of vaporization of carbon disulfide. The volatility of diethyl ether is evident from a vapor pressure more than 20 times that of water at these temperatures.
This equation can be used to calculate the enthalpy of vaporization of a liquid from its. The weak forces also mean that it does not require a large an input of energy to make diethyl ether boil and so it has a relatively low normal boiling point of 34 6 c. Recall that diethyl ether has weak dispersion forces which meant that the liquid has a high vapor pressure. From the plot of vapor pressures vs temperature above estimate the temperature at.
From the plot of vapor pressures vs temperature above estimate the temperature at which the vapor pressure of ethanol is 400 mmhg. A simple measurement involves injecting a little of the liquid into a closed flask connected to a manometer.