Diethyl Ether Structural Formula And Molecular Weight
General properties of diethyl ether c 2 h 5 2 o.
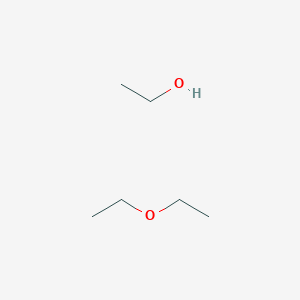
Diethyl ether structural formula and molecular weight. The chemical formula for diethyl ether is c 2 h 5 oc 2 h 5. 12 0107 2 1 00794 5 2 15 9994 percent composition by element. Convert grams diethyl ether to moles or moles diethyl ether to grams. The diethyl ether molecule consists of 10 hydrogen atom s 4 carbon atom s and 1 oxygen atom s a total of 15 atom s.
It is also known by the name ethyl ether. The molecular weight of diethyl ether is determined by the sum of the atomic weights of each constituent element multiplied by the number of atoms which is calculated to be. Ether c2h5 2o or c4h10o cid 3283 structure chemical names physical and chemical properties classification patents literature biological activities. It was formerly used as a general anesthetic until.
Chemsrc provides diethyl ether cas 60 29 7 msds density melting point boiling point structure formula molecular weight etc. Advanced search structure search sort by relevance name name base name base name formula weight formula weight. Cid 3283 ether cid 962 water dates. It is the most common ether known historically.
Cid 3283 ether component compounds. Diethyl ether molecular weight. C 4 h 12 o 2. 116 c tci d3479 116 c alfa aesar 116 c ou chemical safety data no longer updated more details 116 3 c jean claude bradley open melting point dataset 21939 116 c jean claude bradley open melting point dataset 13163 15719 6838 116 c alfa aesar 16767 33224 38990 40976 178 176 f 116 6667 115 5556 c wikidata q202218 177 f 116 1111 c wikidata q202218.
Articles of diethyl ether are included as well. The discovery of ether is credited to the german physician and botanist valerius cordus in 1515 1554. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. C 4 h 10 o.