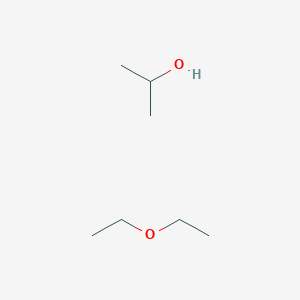
Diethyl Ether Structural Diagram
Combustion ether is highly flammable liquid and undergoes combustion reaction resulting in the formation of carbon dioxide and water.
Diethyl ether structural diagram. Halogenation ether reacts with halogens like chlorine or bromine forming halo substituted ether undergoes substitution reaction in the absence of sunlight. C 2 h 5 oc 2 h 5 6o 2 4co 2 5h 2 o. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. Chemical properties of diethyl ether c 2 h 5 2 o.
Et2o diethylether c8h20o2 cid 21910879 structure chemical names physical and chemical properties classification patents literature biological activities. Diethyl ether ether 60 29 7 et2o. Diethylether water c4h12o2 cid 21584184 structure chemical names physical and chemical properties classification patents literature biological activities. Articles of diethyl ether are included as well.
It was formerly used as a general anesthetic until.