Diethyl Ether Nmr Spectra

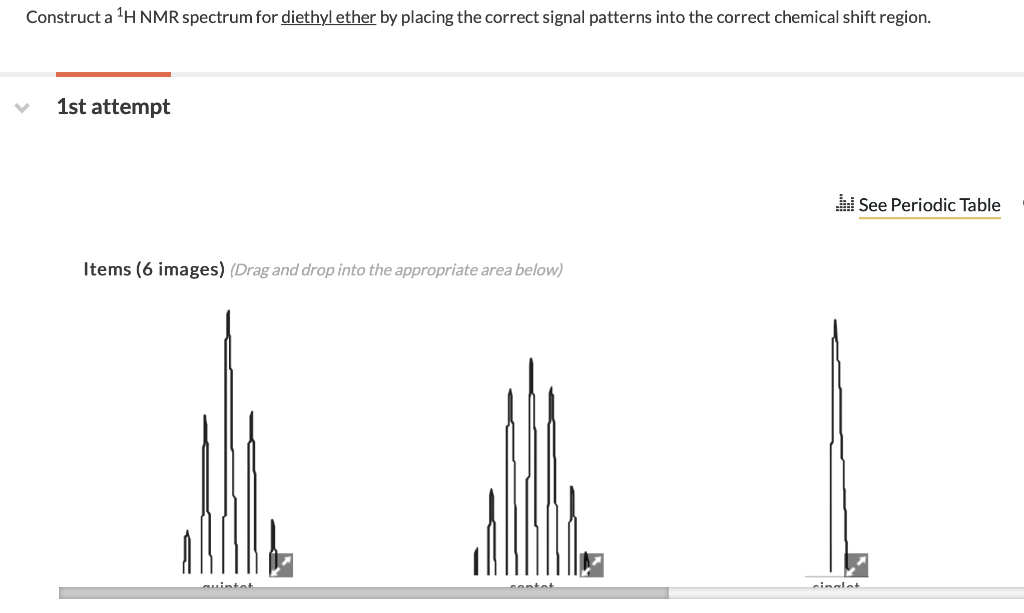
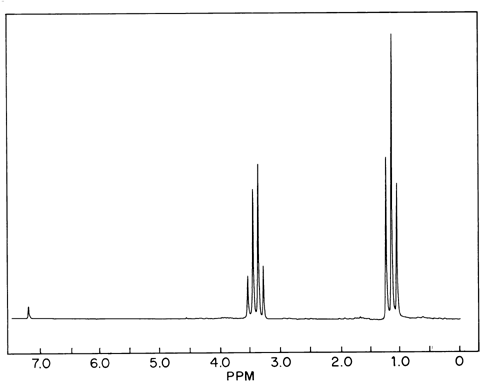
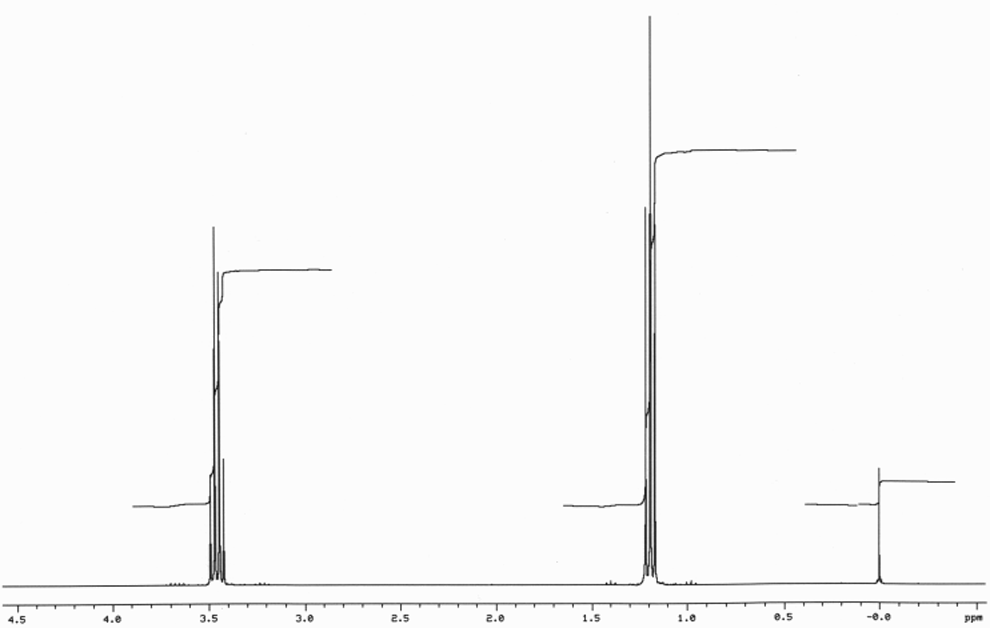
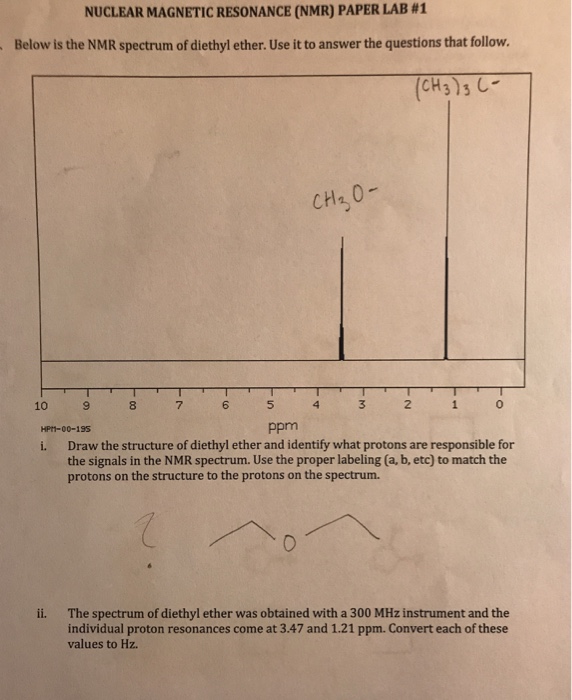
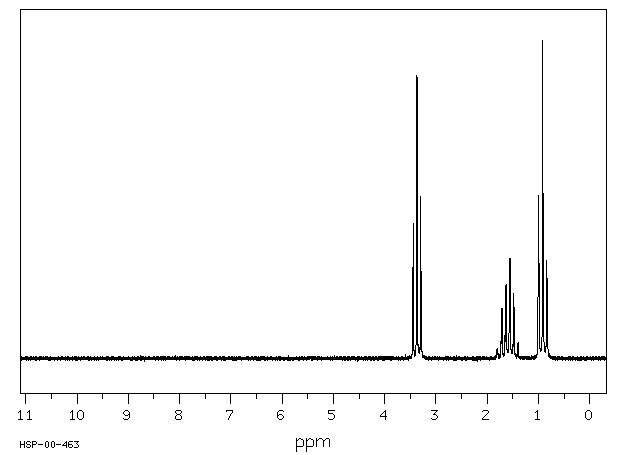
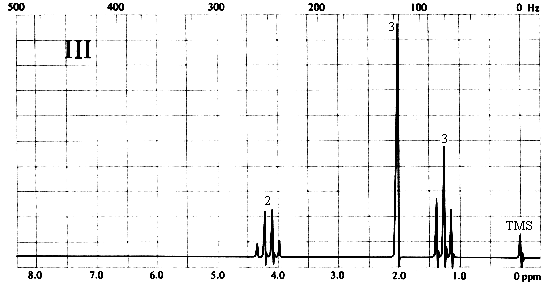
There are four peaks and four carbons.
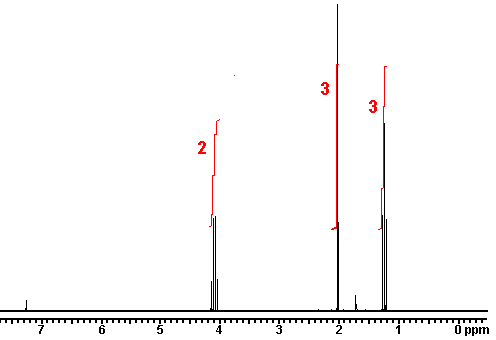
Diethyl ether nmr spectra. No two carbons are in exactly. Two hydrogen atoms give quartet due to adjacent ch 3 group and three hydrogen atoms give triplet due to ch 2 group. The compound is ethoxyethane diethyl ether ch 3 ch 2 och 2 ch 3. Results proton spectra table 1.
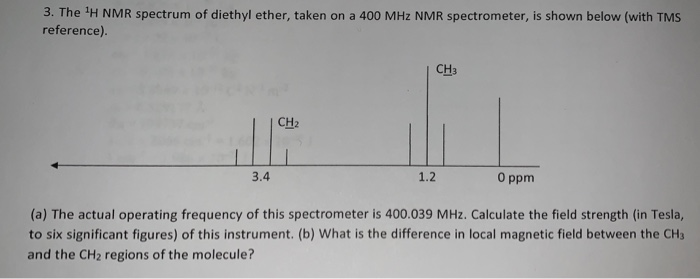
26 nmr 8 ftir and 2 raman. A sample of 0 6 ml of the solvent containing 1 µl of tms 1 was first run on its own. The h nmr spectrum of diethyl ether shows two signals. In d 2oand cd 3od nitromethane was run separately as the protons exchanged with deuterium in presence of triethylamine.
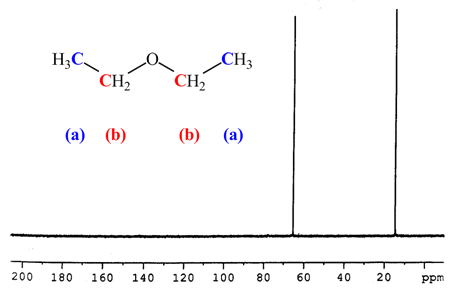
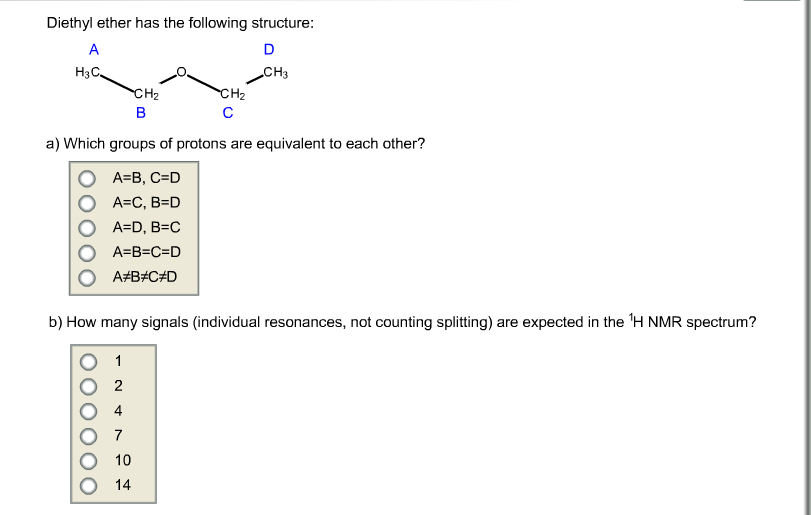
The molecule has a plane of symmetry so the two ch groups are equivalent and the two ch groups are equivalent. Tags diethyl ether 60 29 7 1 h nmr related products ethylparaben 120 47 8 ir1 ethylparaben 120 47 8 ir2 ethylparaben 120 47 8 1 hnmr ethylparaben 120 47 8 13 cnmr ethylparaben 120 47 8 ir3 ethylparaben 120 47 8 ms ethylparaben 120 47 8 raman 2 2 dichlorodiethyl ether 111 44 4 13 cnmr 2 2 dichlorodiethyl ether 111 44 4 raman 2 2 dichlorodiethyl ether 111 44 4 ms 2 2 dichlorodiethyl. The structure of diethyl ether and 2 butanol is shown below. 13c nuclear magnetic resonance nmr chemical shifts of diethyl ether with properties.
From this spectrum we determined the chemical. We should expect the ch groups to be pulled about 2 ppm. This project is supported by the canadian institutes of health research canada foundation for innovation and by the metabolomics innovation centre tmic a nationally funded research and core facility that supports a wide range of cutting edge metabolomic studies tmic is funded by genome canada genome alberta and genome british columbia a not for profit organization that is leading. Its molecular formula is c 4 h 6 o 2.
The effect decreases rapidly with distance. In diethyl ether all carbon atoms are nmr active. Diethyl ether view entire compound with free spectra. Diethyl ether 60 29 7 nmr spectrum diethyl ether h nmr spectral analysis diethyl ether c nmr spectral analysis ect.
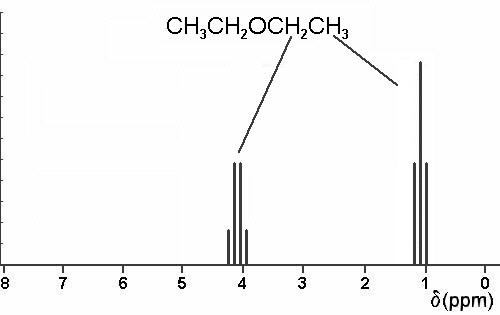
A simple ch 3 in the area of 1 and a ch 2 shifted down to about 4 by the electronegative oxygen. The chemical shift. Proton nmr for a molecule such as diethyl ether ch 3 ch 2 och 2 ch 3 two types of protons would be predicted to appear in the nmr spectrum. Let s sort out what we ve got.
Butyl alcohol diethyl ether tetrahydrofuran. We should see two signals with area ratios 6 4 or 3 2. 26 nmr 8 ftir and 2 raman compound with free spectra. The electronegative o atom pulls all signals downfield.