Diethyl Ether Boiling Point Water

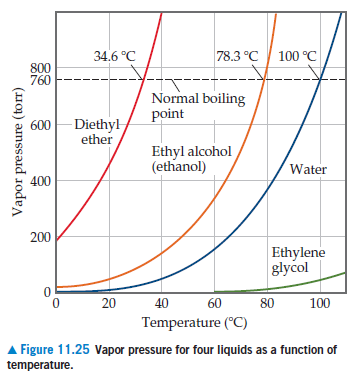
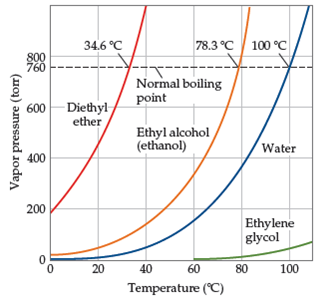
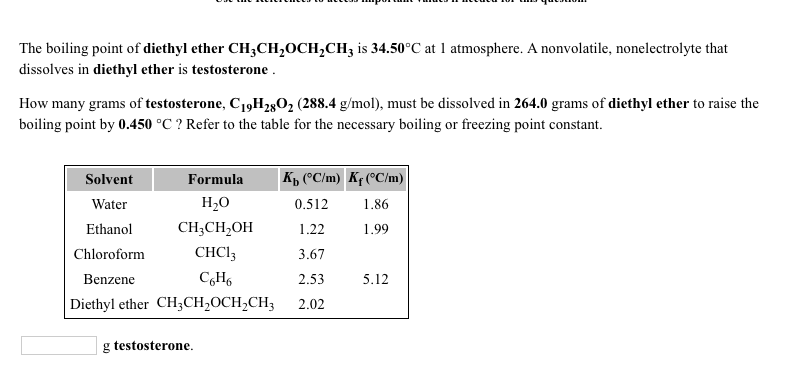
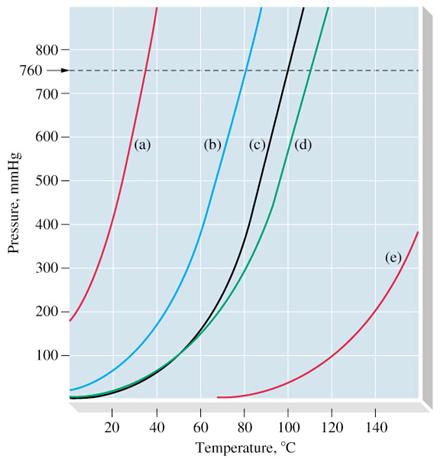
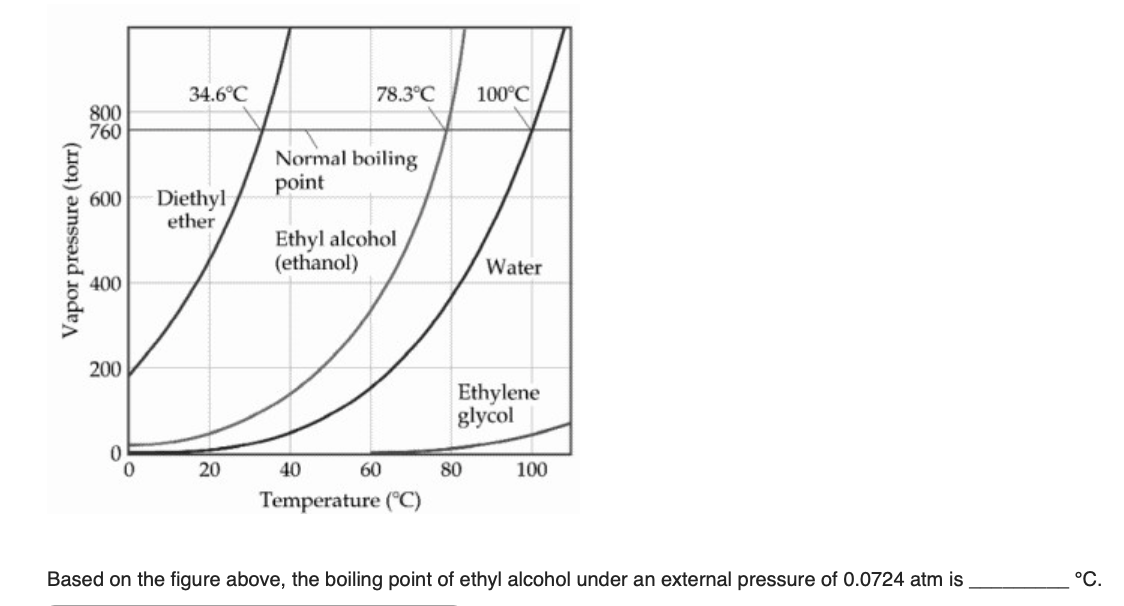
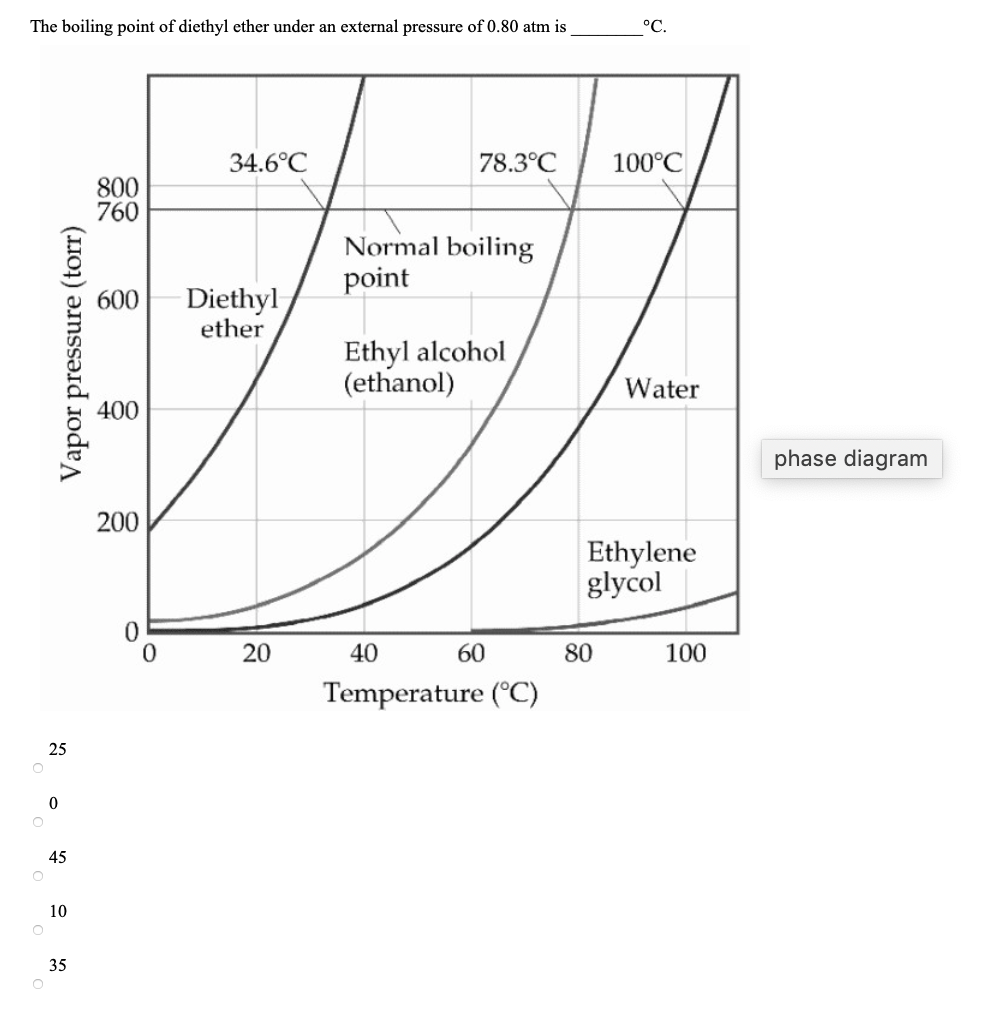
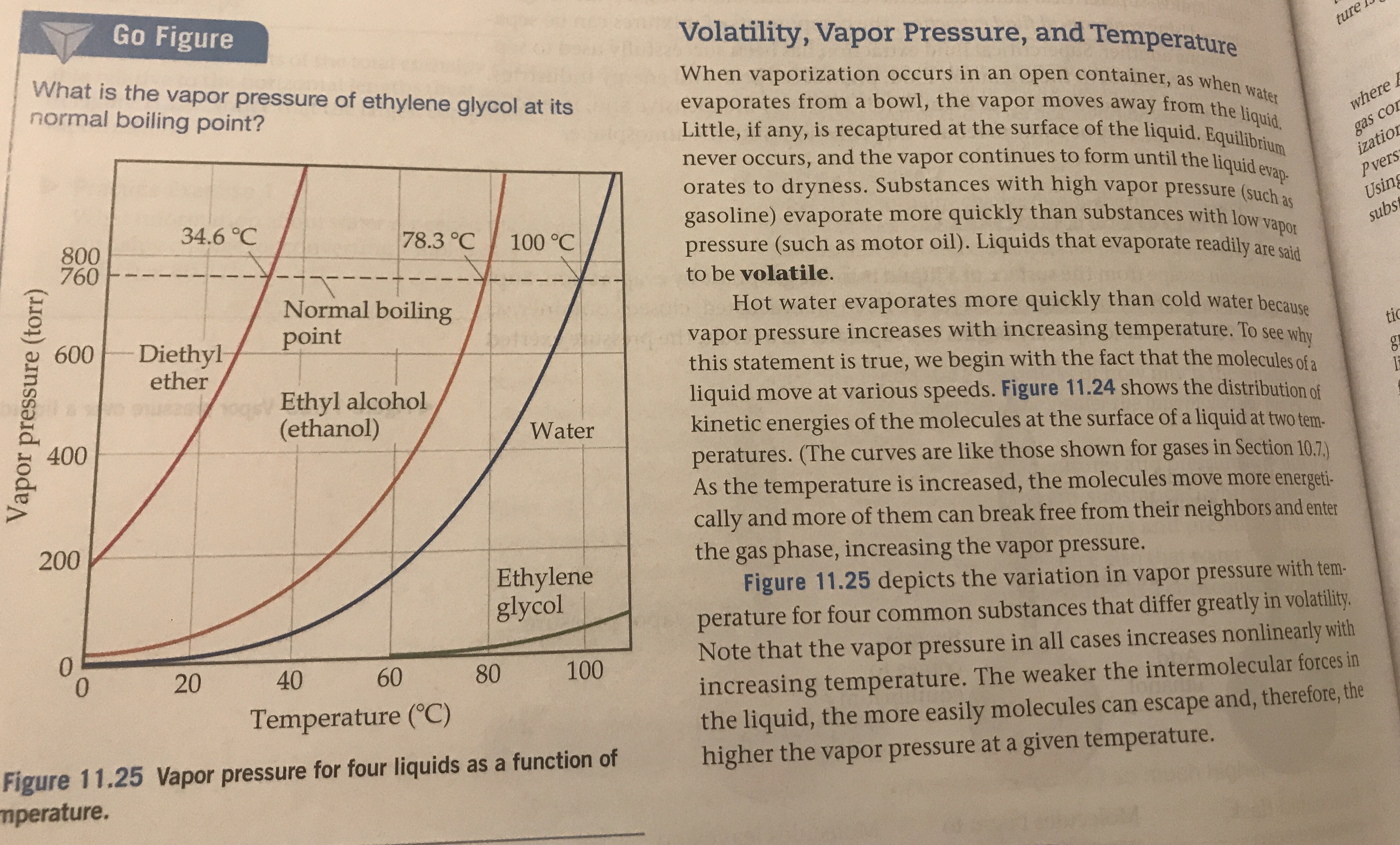
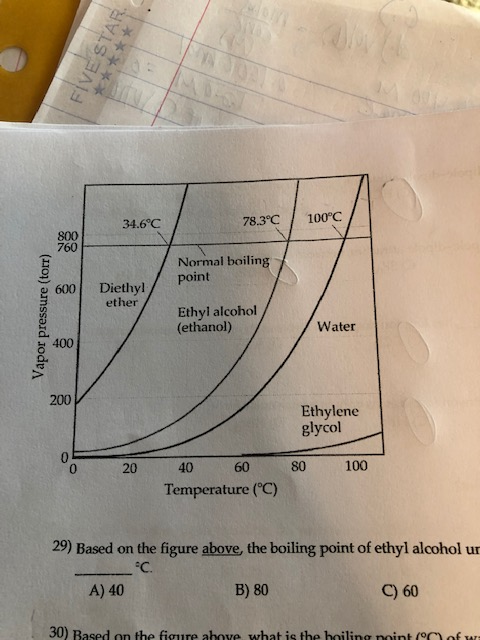
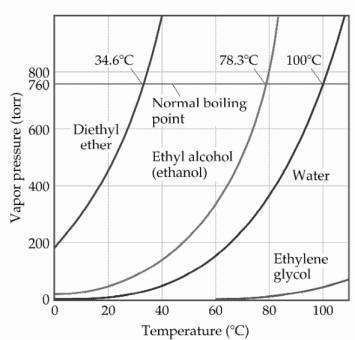
The normal boiling point of diethyl ether is 34 5 c.
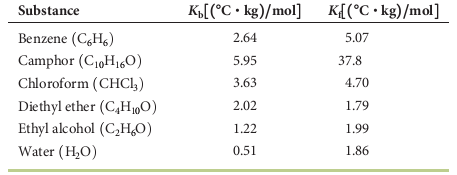
Diethyl ether boiling point water. Diethyl ether has the formula ch 3 ch 2 o ch 2 ch 3. Diethyl ether also known as ether and ethoxyethane is a clear colorless and highly flammable liquid with a low boiling point and a characteristic smell. It is an isomer of butanol. C 2 h 5 oc 2 h 5 6o 2 4co 2 5h 2 o.
Combustion ether is highly flammable liquid and undergoes combustion reaction resulting in the formation of carbon dioxide and water. Citation needed ether is sensitive to light and air tending to form explosive peroxides. Articles of diethyl ether are included as well. Halogenation ether reacts with halogens like chlorine or bromine forming halo substituted ether undergoes substitution reaction in the absence of sunlight.
Diethyl ether cas 60 29 7 is a component of starting fluids and is used as a solvent in the manufacture of synthetic dyes and plastics. Monticelli in encyclopedia of toxicology third edition 2014. Ether peroxides have a higher boiling point than ether and are contact explosives when dry. Ether c2h5 2o or c4h10o cid 3283 structure chemical names physical and chemical properties classification patents literature biological activities.
Combustion ether is highly. It is the most common member of a class of chemical compounds known generically as ethers. Chemsrc provides diethyl ether cas 60 29 7 msds density melting point boiling point structure formula molecular weight etc. Because of its characteristics diethyl ether was widely used in many countries as an anesthetic agent but was then replaced by other substances in the 1960s.
Chemical properties of diethyl ether c 2 h 5 2 o.