Benzoic Acid And Diethyl Ether Reaction Equation

Get the detailed answer.
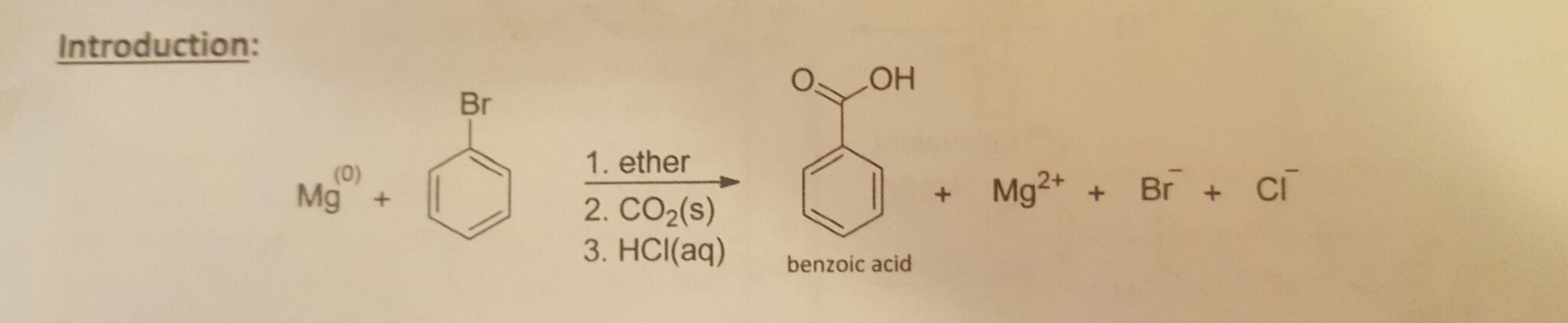
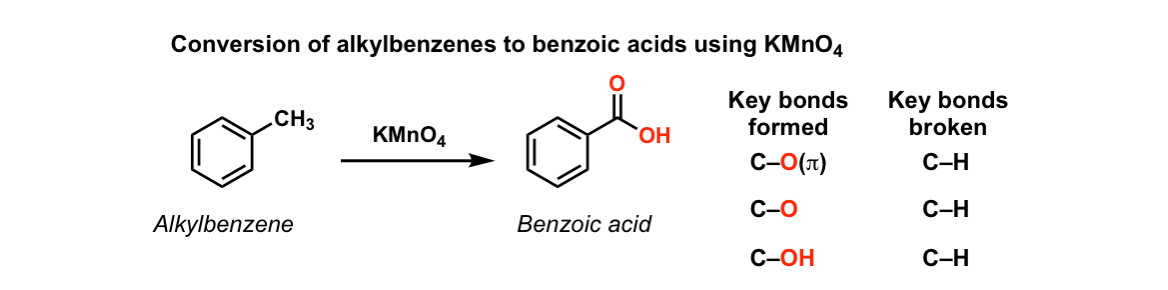
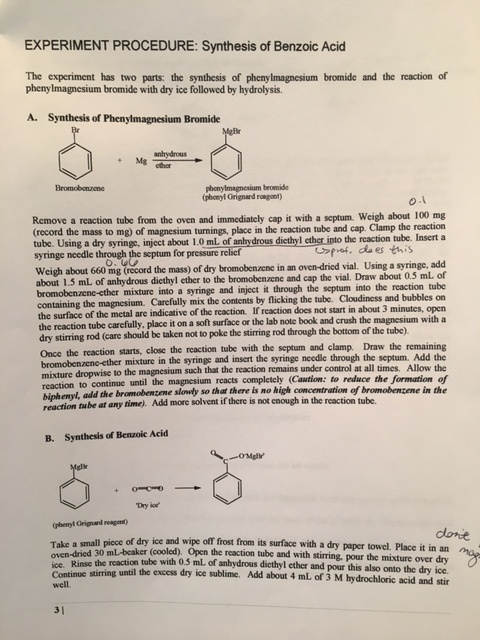
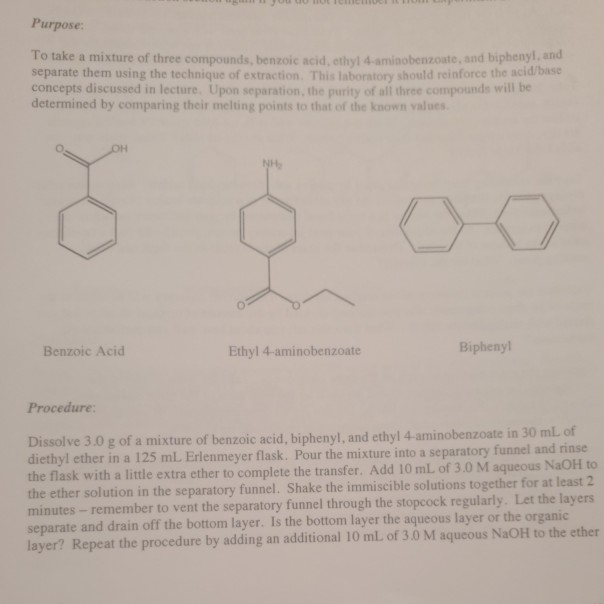
Benzoic acid and diethyl ether reaction equation. A solution of benzoic acid in 5 naoh is placed in a flask. A complete the acid base reaction below by writing the products of the reaction. Bromobenzene and diethyl ether is used in the formation of g. In this reaction the grignard reagent an organomagnesium compound phenylmagnesium bromide is prepared by reaction of bromobenzene with magnesium metal in diethyl ether the solvent.
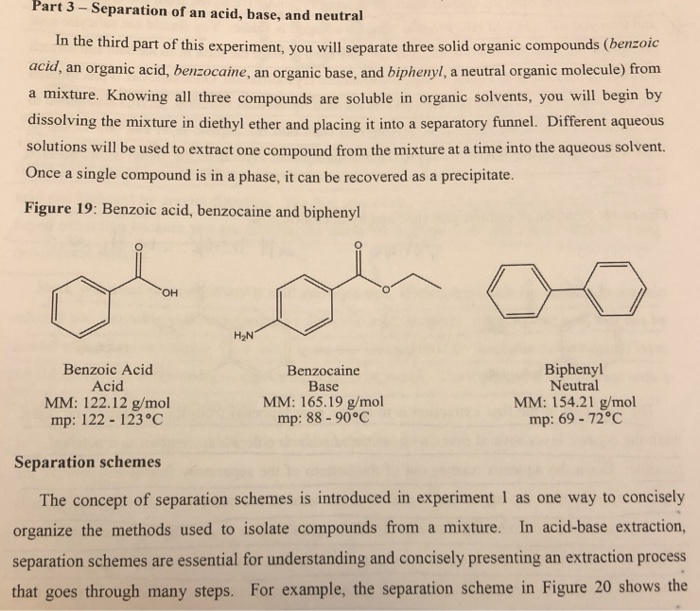
An ether layer and an aqueous layer. Grignard reagent formation and reactions. Ether is immiscible with aqueous solvents and the mixture of liquids forms 2 layers. Please consider the preperation of benzoic acid.
The grignard reagent will then be converted to benzoic acid via the reaction of the grignard reagent with excess dry ice solid co 2. Bromobenzene is a skin irritant. For more about diethyl ether and benzoic acid reaction please subscribe to our website newsletter now. An equal volume of diethyl ether is then added and the mixture is stoppered shaken and then allowed to stand to reach equilibrium.
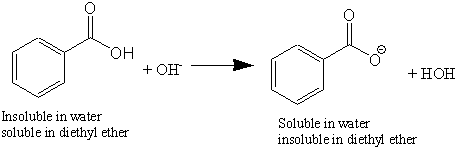
B in the reaction in part a label the acid the base the conjugate acid and conjugate base. Get to know more about ketogenic diet and diethyl ether and benzoic acid reaction here on this site. Avoid breathing bromobenzene or ether fumes. A which liquid forms the top layer i believe it s ether because it s less dense.
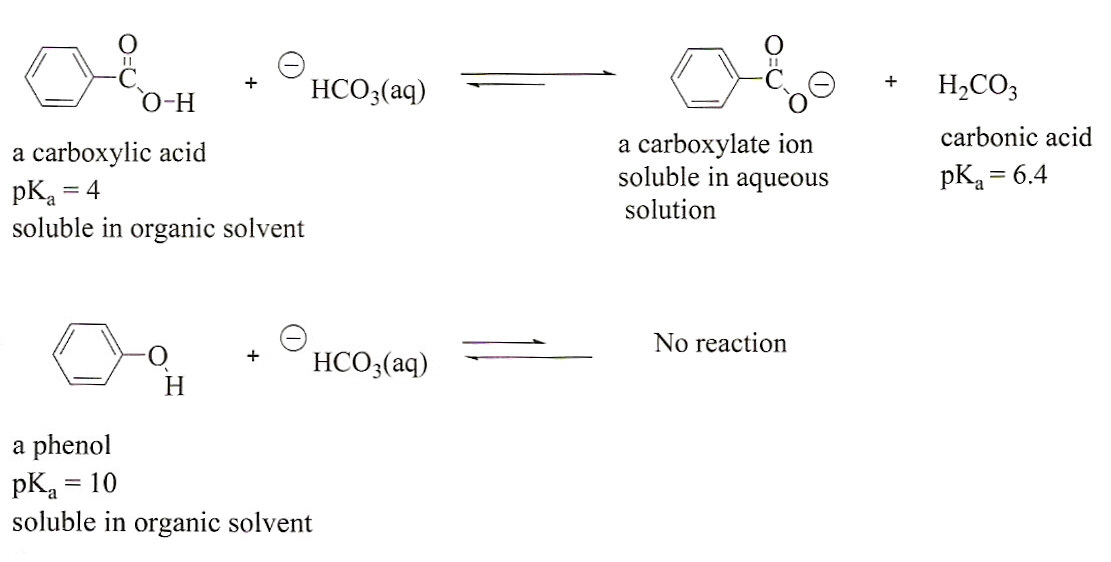
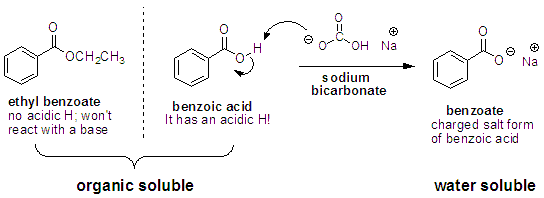
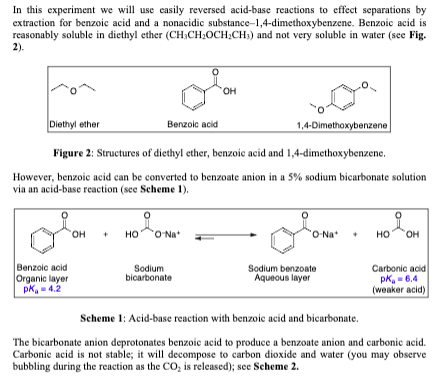
First both molecules have a portion that is non polar the benzene ring in benzoic acid the two ethyl groups in diethyl ether the second reason is the hydrogen bonding that can occur between the proton on the carboxyl group of benzoic acid and the ether oxygen of diethyl ether. There must be no flames or sparking sources present in the laboratory during this experiment. C indicate the. Safety precautions diethyl ether is extremely volatile and flammable.
Wear gloves goggles and a lab coat to avoid skin.