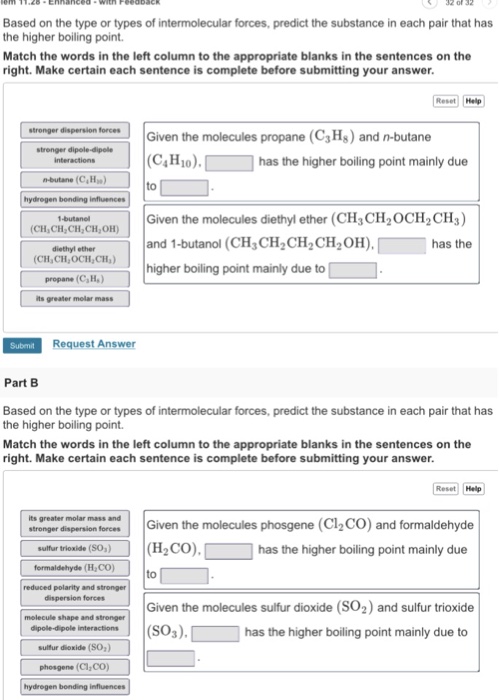
Why Diethyl Ether Has Higher Boiling Point Than Butane
Its boiling point is 35 o c.
Why diethyl ether has higher boiling point than butane. In the case of diethyl ether the molecules are held together by dipole dipole interaction which arises due to the polarized c o bond. 1 butan 1 ol has higher boiling point than diethyl ether because butan 1 ol has strong intermollecular hydrogen bonding but in diethylether they are associated with weak dipole dipole attractions. Diethyl ether and 1 butanol are similar in size number of electrons therefore their boiling points will be determined by polarity. Compare its boiling point.
2 mechanism of. Diethyl ether has two polar c o bonds. The ethanol has a much higher boiling point. 1 butanol ch3ch2ch2ch2oh and diethyl ether ch3ch2och2ch3 both have the same molecular formula but the boiling point of 1 butanol is.
Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. A group of ethanol molecules is much harder to separate from each other than a group of dimethyl ether molecules. It was formerly used as a general anesthetic until. But in case of ether the hydrogen is linked to c and it is not so electronegative to encourage the hydrogen to form hydrogen bonding.
1 butanol also has. Why butanol has higher boiling point than diethylether 2 see answers.