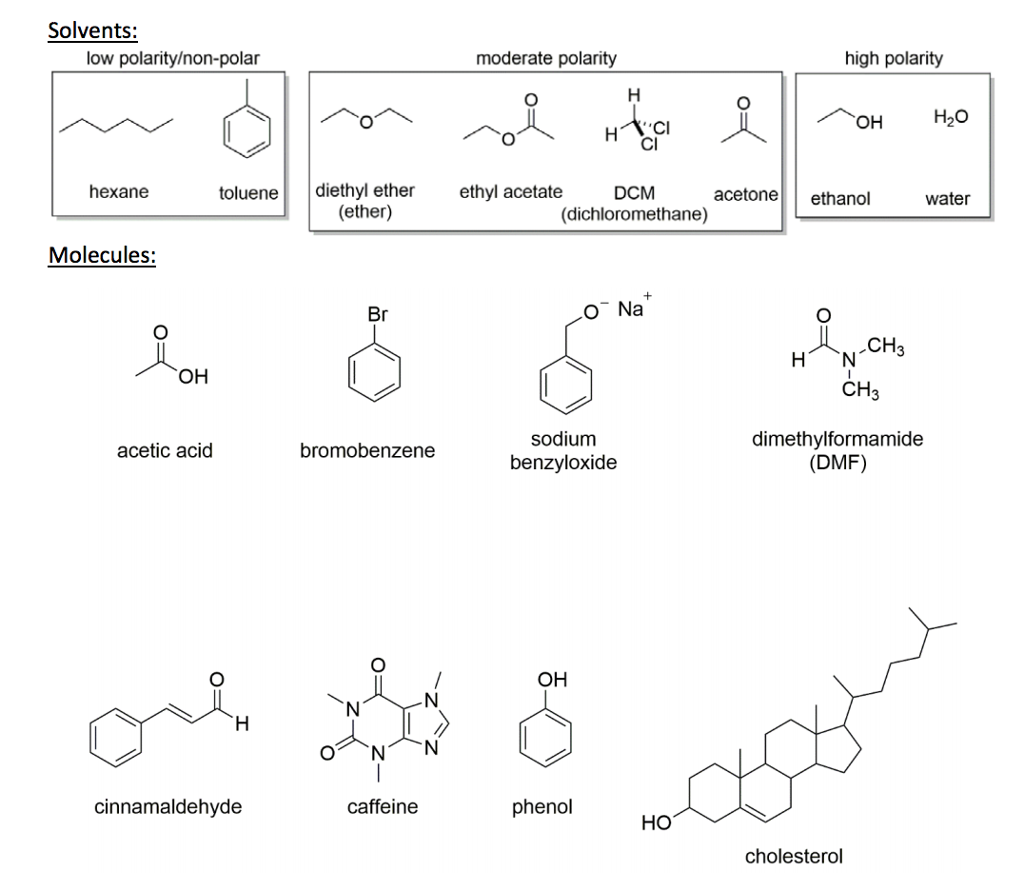
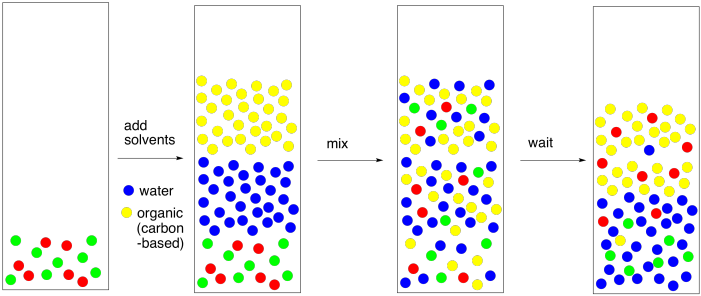
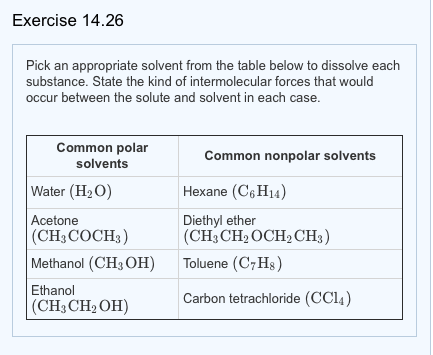
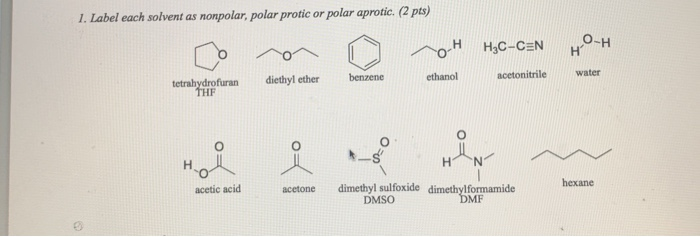
Water Or Diethyl Ether Polar
Oxygen is more electronegative than h hence attract pair of electrons in the bond.
Water or diethyl ether polar. Electro negativity difference between carbon and oxygen is less hence less attraction of pair of electrons in the c o bond. O becomes slightly negative and h slightly positive. Ethoxyethane or diethyl ether is a polar molecule as the oxygen in the middle of the molecule forms two polar bonds with the carbons on both sides. Diethyl ether being a symmetrical molecule theb dipole moments directed toward the ethrial o atom cancel out with each other making the molecule non polar almost thf on the other hand is not non.
Cid 3283 ether. The oxygen has two lone pairs that can fo. Could you please help for the tips i would like to know whether ethyl ether or other name diethyl ether is the non polar is because i am interested to use it for extraction of wet algae. Topological polar surface area.
Is diethyl ether c2h5 2o or ch3ch2och2ch3 polar or nonpolar. When the ph of a solution that has two layers and contained benozic acid is altered from neutral to basic how is the solubility of benzoic acid in the water layer affected. Water is a polar protic solvent such that the water molecules stabilize the activity of any anion or cation dissolved in that solution. By changing the ph of the solution from neutral to basic the polarity of the benzoic acid changes from nonpolar to polar and thus this change in polarity.
Diethyl ether is c2h5 oc2h5 that is a link of c o c. With other solvents say diethyl ether or ammonia or even hexanes the activity of the anion may be greatly magnified and thus this solvent is used as the medium for the reaction. How many grams of sodium hydroxide are required to make 1 liter of a 3m solution of naoh. Diethyl ether c2h5 2o or ch3ch2och2ch3 is nonpolarnonpolar.
Water is more polar than diethyl ether and this is because the electronegative difference between oxygen and hydrogen is greater than the electronegative difference of carbon and oxygen thus making water the more polar substance.