Hybridization Of Diethyl Ether
Methyl phenyl ether is anisole because it was originally found in.
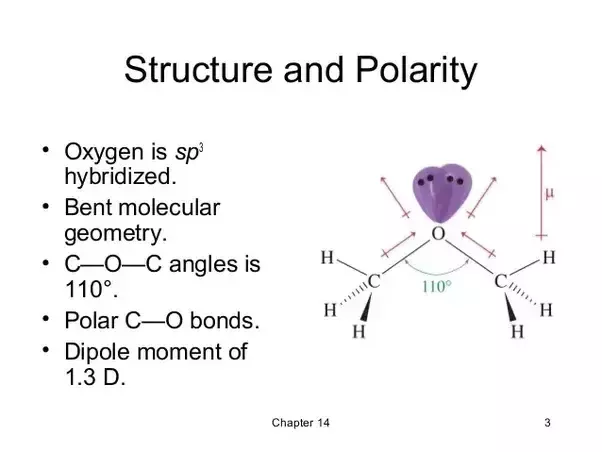
Hybridization of diethyl ether. They did not use the sp2 hybridsation model with an sp2 and 2p orbital lone pair on the oxygen as outlined in the paragraph. In other words the electrons are distributed unevenly which causes high and low areas of electron density. Sp which product would be formed from a williamson synthesis when reacting propyl iodide with ethanol. Diethyl ether is simply called ether but was once called sweet oil of vitriol.
Ether bonds are quite stable towards bases oxidizing agents and reducing agents. But on the other hand ethers undergo cleavage by reaction with acids. After hybridization these five electrons are placed in the four equivalent sp 3 hybrid orbitals. So this molecule is diethyl ether and let s start with this carbon right here so the hybridization state.
In case of dimethyl ether oxygen atom is sp3 hybridised. Diethyl ether s dielectric constant is 4 3 so some consider it to be non polar. For example ethyl methyl ether ch 3 oc 2 h 5 diphenylether c 6 h 5 oc 6 h 5. Diethyl ether ch3 ch2 o ch2 ch3 has.
Removing all traces of water to form a solid. Due to the sp3 hybridization the nitrogen has a tetrahedral geometry. Of all the functional groups ethers are the least reactive ones. When comparing to other compounds containing a c o c bond i came across diethyl ether.
In ether oxygen atom is sp 3 hybridized with a bond angle of 109 5 0 ether. In furan oxygen atom contains two bonds wit. However others argue that diethyl ether is polar due to the geometry of the molecule that does not cause the oxygen carbon bond dipoles to cancel. But if we take an example of furan then oxygen atom is sp2 hybridised.
Diethyl ether propyoxyethane cyclopentene cyclopentane to what does the term drying a liquid refer. Diethyl ether would have two lone pairs of electrons and would have a bent geometry around the oxygen. Oxygen atom has two bonds with ch3 group and two lone pair of electrons. 2 sp3 at the ch3 3 bonds to hydrogens and one bond to a carbon 2 sp3 at the ch2 it is bonded to a carbon oxygen and hydrogens sp3 at the o two lone pairs and two bonds to carbonds all of the bonds are sigma bonds.
Diethyl ether followed my reasoning in which 4 sp3 hybrids were formed in the oxygen involved and a model as explained above was used. Dimethyl amine would have one lone pair and would show a pyramidal geometry around the nitrogen. Diethyl ether would have two lone pairs of electrons and would have a bent geometry around the oxygen. Well the fast way of doing it is to notice that there are only single bonds around that carbon only sigma bonds and so therefore we know that carbon is sp three hybridized with tetrahedral geometry so sp three hybridized.