Dimethyl Ether Vapor Pressure
Vapor pressures of dimethyl ether were measured at temperatures from 233 to 399 k and at pressures from 54 to 5146 kpa.
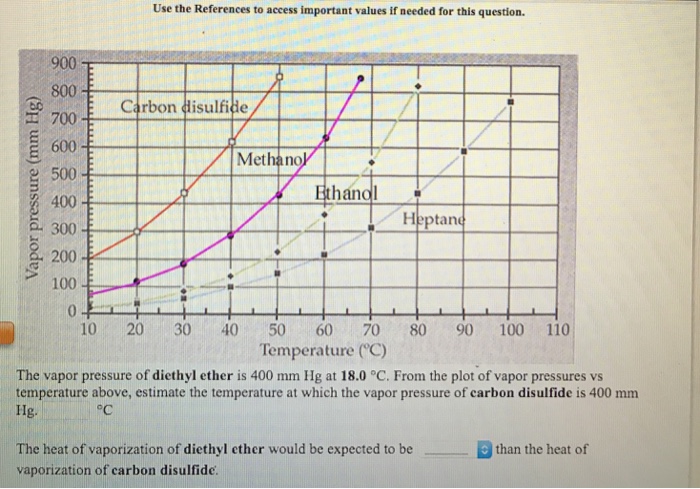
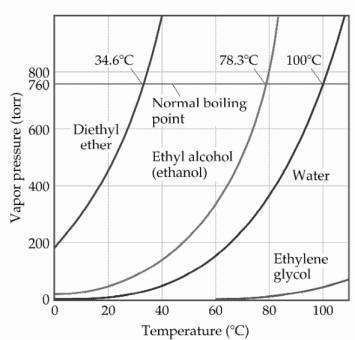
Dimethyl ether vapor pressure. Any leak can be either liquid or vapor. Why does dimethyl ether have a higher vapor pressure than ethanol at a given temperature. The weak forces also mean that it does not require a large an input of energy to make diethyl ether boil and so it has a relatively low normal boiling point of 34 6 c. With the new experimental data a wagner type vapor pressure equation of dimethyl ether was fitted.
It is an isomer of ethanol. It can asphyxiate by the displacement of air. Vapor pressure of dimethyl ether was measured using a modified constant volume apparatus. Recall that diethyl ether has weak dispersion forces which meant that the liquid has a high vapor pressure.
Dimethyl ether has the formula ch3och3 and ethanol has the formula ch3ch2oh. Its vapors are heavier than air. A total of 39 experimental points were obtained with uncertainties of 5 mk on its 90 for temperature and 0 7 kpa for pressure. All data.
Dimethyl ether has the formula ch3och3 and ethanol has the formula ch3ch2oh. The experimental data shown in these pages are freely available and have been published already in the ddb explorer edition the data represent a small sub list of all available data in the dortmund data bank for more data or any further information please search the ddb or contact ddbst. Dimethyl ether dme also known as methoxymethane is the organic compound with the formula ch3och3 simplified to c2h6o. It is shipped as a liquefied gas under its vapor pressure.
The measurement results were fitted to a wagner type equation with. 1941 63 2267 2272. The simplest ether it is a colorless gas that is a useful precursor to other organic compounds and an aerosol propellant and is being studied as a future energy option. Contact with the liquid can cause frostbite.
Dimethyl ether is a colorless gas with a faint ethereal odor. The heat capacity and entropy heats of fusion and vaporization and the vapor pressure of dimethyl ether. Why does dimethyl ether have a higher vapor pressure than ethanol at a given temperature. It is easily ignited.
Vapor pressure of diethyl ether. Measurements were carried out at temperatures from 213 to 393 k and at pressures from 16 to 4706 kpa. The density of gaseous dimethyl ether j.