Dimethyl Ether Polar Nonpolar
Chcl3 chloroform or trichloromethane.
Dimethyl ether polar nonpolar. Ch4o methanol polar. Here s the best image i could fi. There is very little intermolecular association because the carbon hydrogen bond is non polar. Could you please help for the tips i would like to know whether ethyl ether or other name diethyl ether is the non polar is because i am interested to use it for extraction of wet algae.
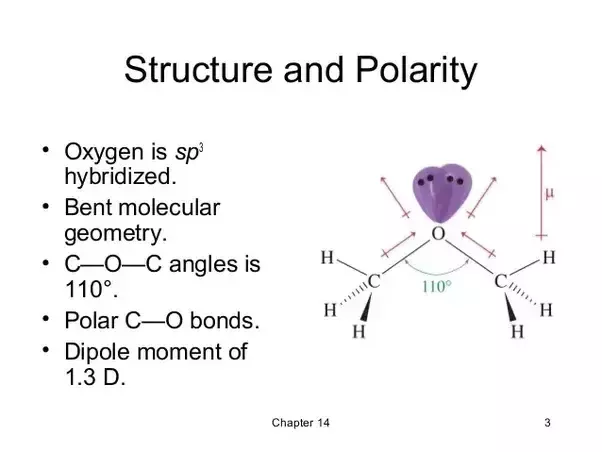
Each of the c o bonds are polarised as shown in the figure. Ch3och3 dimethyl ether polar. Dimethyl ether dme also known as methoxymethane is the organic compound with the formula ch 3 och 3 simplified to c 2 h 6 o. Question is clf polar or nonpolar.
For many purposes yes. S ch3 2 is the formula for dimethyl sulfide analogous to dimethyl ether. Answer ch3och3 dimethyl ether is polar what is polar and non polar. Petroleum ether is nonpolar.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Question is ch3och3 polar or nonpolar. Dimethyl ether contains two methyl groups and has a bent structure. C3h6o or ch3 2co or ch3coch3 acetone diethyl ether c2h5 2o or ch3ch2och2ch3.
I m not sure what context your question is in. Alkanes alkenes and alkynes are essentially non polar and insoluble in water. The distinction polar and nonpolar is really not that informative because the important thing to remember is that everything is relative. What is the formula of s ch3 2.
But in practice it is considerably less polar than water and in fact is mostly insoluble in it. The hydrogen oxygen and carbon oxygen bonds are polar covalent bonds. Diethyl ether is polar relative to other molecules such as hexane. Is petroleum ether polar or nonpolar.
By bagus amin januari 29 2018 add comment. But this is a matter of degree. The simplest ether it is a colorless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applications it is an isomer of ethanol. Ethers are essentially non polar and insoluble in water.
In addition to this the lone pair polarisation also contributes to its dipole moment. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Polar in chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.