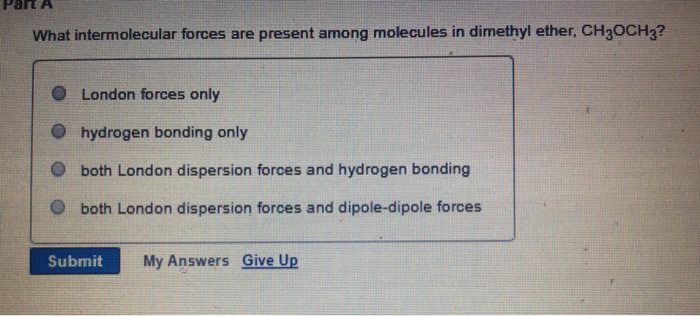
Dimethyl Ether Dominant Intermolecular Forces
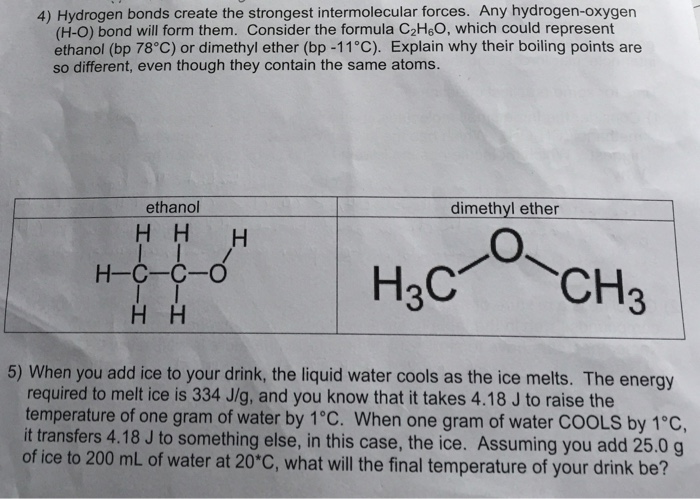
Chapter 10 intermolecular forces 25.
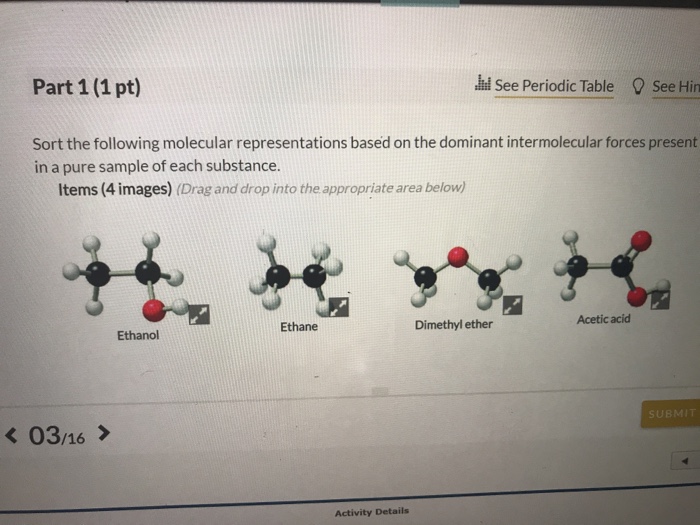
Dimethyl ether dominant intermolecular forces. The van der waals forces encompass intermolecular forces as well as some intramolecular forces including keesom interaction the debye force and the london dispersion force. D c 2 h 4 or ch 3 oh. B c 2 h 5 oc 2 h 5 diethyl ether or c 4 h 9 oh butanol. Dispersion dipole dipole and or hydrogen bonding the answer may have one two or all three of the choices but i already tried all three and it was wrong.
Explain which substance in each of the following pairs is likely to have the higher normal melting point. It is shipped as a liquefied gas under its vapor pressure. The forces are used to explain the universal attraction between bodies the physical adsorption of gases and the cohesion of condensed phases. Identify the types of intermolecular forces present in ch3och3.
It can asphyxiate by the displacement of air. London dispersion forces d. When c4h10o diethyl ether interacts with h2o water there are three intermolecular forces. Nacl because it is an ionic compound not molecular.
Dimethyl ether 46 07 g mol mp 139 c bp 25 c density 0 00195 g mol 20 c refrigerant coh h h h h. C8h18 hooh nh2nh2 hssh and ch3ch3. It is easily ignited. Dipole dipole interactions london dispersion forces or hydrogen bonding.
Contact with the liquid can cause frostbite. In contrast to intramolecular forces such as the covalent bonds that hold atoms together in molecules and polyatomic ions intermolecular forces hold molecules together in a liquid or solid intermolecular forces are generally much weaker than covalent bonds. Dimethyl ether cannot. 22 removes dark cloud for uber and lyft michael j.
Butanol due to hydrogen bonding in butanol. Compound b has an rf value of 42 in hexane and 60 in diethyl ether. C chi 3 or chf 3. The properties of liquids are intermediate between those of gases and solids but are more similar to solids.
The positive h of h2o is attracted to the negative o of c4h10o. A hcl or nacl. In solids the intermolecular forces are so strong that the particles are held rigidly in place. Any leak can be either liquid or vapor.
Dimethyl ether is a colorless gas with a faint ethereal odor. The rf value of compound a is 34 when the tlc plate is developed in hexane and 44 when the plate is developed in diethyl ether. Chapter 10 intermolecular forces 3.