Dimethyl Ether And Ethanol Boiling Point
And the answer is an hydrogen atom directly bound to a strongly electronegative atom such as nitrogen or fluorine or her.
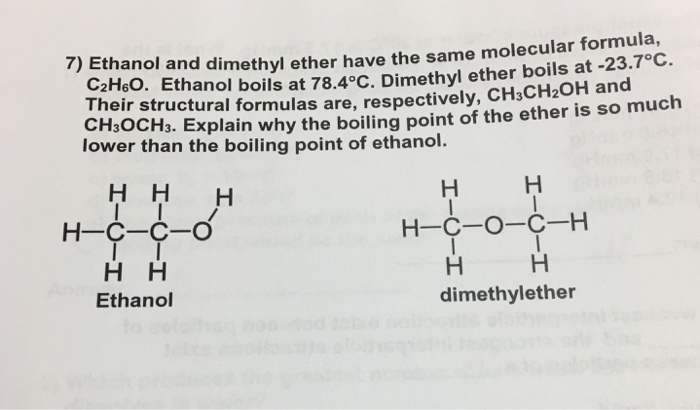
Dimethyl ether and ethanol boiling point. Boiling point of ethanol is 78 37 c. Dimethyl ether dme also known as methoxymethane is the organic compound with the formula ch 3 och 3 simplified to c 2 h 6 o. This is because in ethanol the hydrogen and the oxygen are bonded which results in a large electronegativity difference. Dimethyl ether is an ether.
Between dimethyl ether ch3 o ch3 and ethanol ch3 ch2 oh the la er has a higher boiling point because a. The boiling point of any liquid can be defined as the minimum temperature at. Ch3ch2oh 78 oc is higher than the boiling point of. This allows hydrogen bonding to occur.
The simplest ether it is a colorless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applications it is an isomer of ethanol. Well what has ethanol math h 3 cch 2 oh math got that dimethyl ether i e. Ethanol and dimethyl ether have the same molecular formula c2h6o but the boiling point of ethanol ch3ch2oh 78 oc is higher than the boiling point of dimethylether ch3 2o 25 oc because a of hydrogen bonding b of stronger dipole dipole interactions c of larger london dispersion forces d it has a larger molar mass e the bonds are more polar enter the letter of the correct. It has a linear geometry.
Ethanol is an alcohol. Dimethyl ether ch 3 och 3 and ethanol c 2 h 5 oh have the same formula c 2 h 6 o but the boiling point of dimethyl ether is 25 c while that of ethanol is 78 c. B of stronger dipole dipole interactions. Boiling point of dimethyl ether is 24 c.
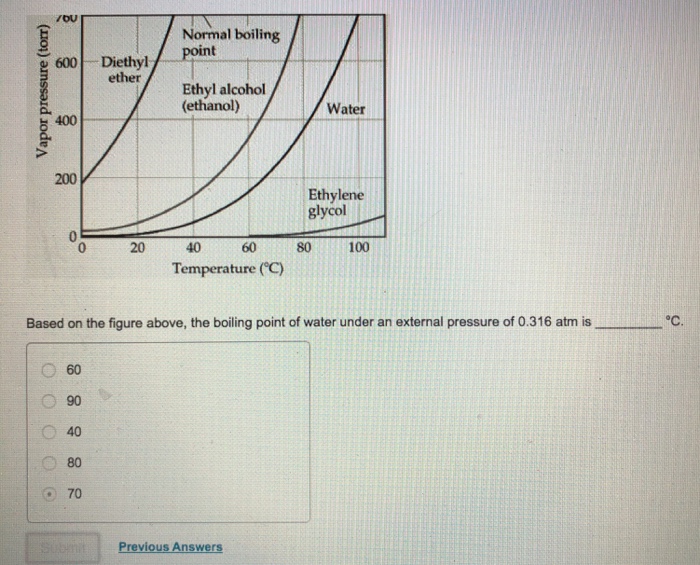
The melting point of ethanol is 114 1 c. What are the boiling points of ethanol dimethyl ether propane water and methyl alcohol. It has high dipole dipole interacwon. It has a larger molecule.
Ethanol and dimethyl ether have the same molecular formula c2h6o but the boiling point of ethanol. Dimethylether ch3 2o 25 oc because a of hydrogen bonding. It has hydrogen bonding. Ethanol and dimethyl ether have the exact same formula but ethanol s boiling point is 78c and dimethyl ether is 25c.
The melting point of dimethyl ether is 141 c.