Diethyl Ether Dissolved In Water
B when 1 phenylcyclohexanol is partitioned between diethyl ether and water in which layer would you expect it to be more soluble.
Diethyl ether dissolved in water. Which is the optimal solvent to extract caffeine from an aqueous solution. Which layer is the top layer and which is the bottom layer. Combustion ether is highly flammable liquid and undergoes combustion reaction resulting in the formation of carbon dioxide and water. Given that mathrm c 6 math.
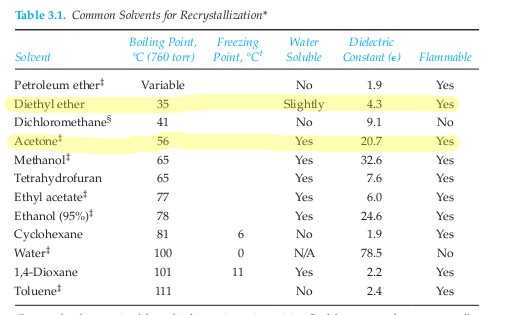
If the exctract is not a solid rather like a. Sulphur dioxide saturated aqueous solution. One gram of caffeine dissolves in 55 ml of water 7 ml of dichloromethane 530 ml of diethyl ether and 100 ml of benzene. Rhodamine b solution dissolve 0 1 g of the solid reagent in 100 ml of 3 5 m hydrochloric acid.
Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols it is a colorless highly volatile sweet smelling ethereal odour flammable liquid it is commonly used as a solvent in laboratories and as a starting fluid for some engines. You approximate that the. The mole fraction equilibrium solubility of nicotinic acid in six solvents water ethanol dimethyl sulfoxide acetone acetonitrile and diethyl ether differing in polarity polarizability and hydrogen bonding ability was determined over the temperature range 283 to 333 k using the gravimetric method. It was formerly used as a general anesthetic until.
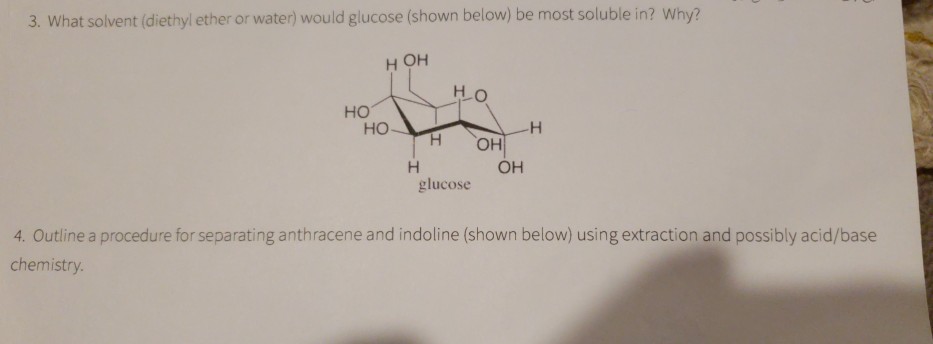
Water and diethyl ether are immiscible liquids. After separating the layers 240 mg of cyclohexanol are recovered from the diethyl ether layer. Explain how you know. Charged compounds dissolve in water and uncharged compounds dissolve in ether.
C 2 h 5 oc 2 h 5 6o 2 4co 2 5h 2 o. Halogenation ether reacts with halogens like chlorine or bromine forming halo substituted ether undergoes substitution reaction in the absence of sunlight. Solution for c diethyl ether.