Diethyl Ether Carbon Nmr
Ethoxyethane diethyl ether ch 3 ch 2 och 2 ch 3.
Diethyl ether carbon nmr. It was formerly used as a general anesthetic until. C from ch 3 8 25 ppm. Tags diethyl ether 60 29 7 13 c nmr related products ethylparaben 120 47 8 ir1 ethylparaben 120 47 8 ir2 ethylparaben 120 47 8 1 hnmr ethylparaben 120 47 8 13 cnmr ethylparaben 120 47 8 ir3 ethylparaben 120 47 8 ms ethylparaben 120 47 8 raman 2 2 dichlorodiethyl ether 111 44 4 13 cnmr 2 2 dichlorodiethyl ether 111 44 4 raman 2 2 dichlorodiethyl ether 111 44 4 ms 2 2 dichlorodiethyl. The structure of diethyl ether and 2 butanol is shown below.
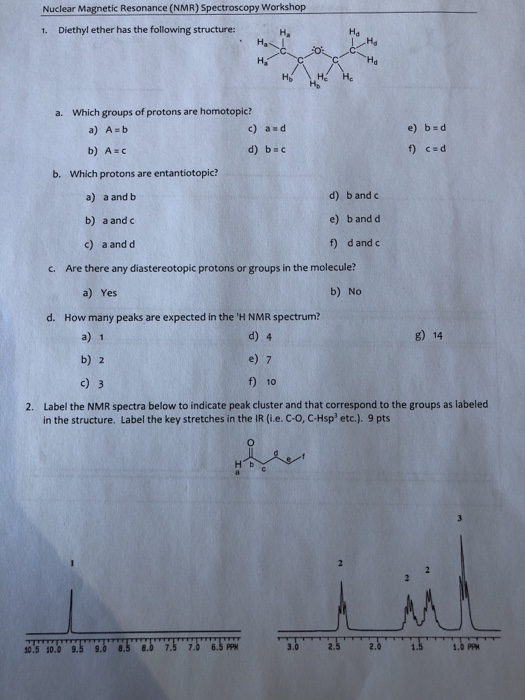
Butyl alcohol diethyl ether tetrahydrofuran. The 1 1 and 2 1 complexes of diethyl ether with tin tetrachloride and their stability studied by 119sn nmr spectroscopy journal of the chemical society perkin transactions 2 2001. In d 2oand cd 3od nitromethane was run separately as the protons exchanged with deuterium in presence of triethylamine. Two hydrogen atoms give quartet due to adjacent ch 3 group and three hydrogen atoms give triplet due to ch 2 group.
Ether c2h5 2o or c4h10o cid 3283 structure chemical names physical and chemical properties classification patents literature biological activities. 13 c nmr spectra provide information about. The chemical shift. Diethyl ether 60 29 7 nmr spectrum diethyl ether h nmr spectral analysis diethyl ether c nmr spectral analysis ect.
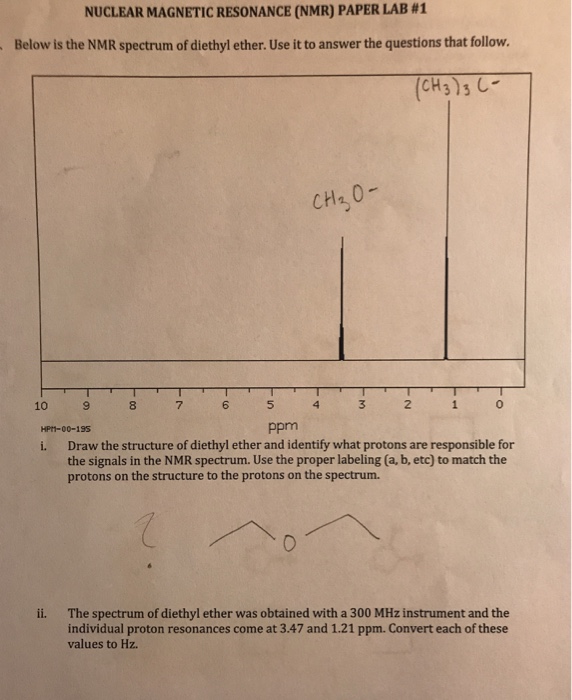
Tables of 1h and 13c nmr chemical shifts have been compiled for common organic compounds. Proton nmr for a molecule such as diethyl ether ch 3 ch 2 och 2 ch 3 two types of protons would be predicted to appear in the nmr spectrum. However due to the symmetry of the molecule only two peaks are observed in nmr spectra. 13c nuclear magnetic resonance nmr chemical shifts of diethyl ether with properties.
In diethyl ether all carbon atoms are nmr active. The nmr spectrum of diethyl ether however displays seven peaks as. 13 c nmr spectroscopy carbon nuclear magnetic resonance spectroscopy is used to identify the structure of organic carbon compounds. Return to nmr home page.
Use the back arrow to return to a spectroscopy problem. A simple ch 3 in the area of 1 and a ch 2 shifted down to about 4 by the electronegative oxygen.